COAPT: MitraClip Reduces Repeat Hospitalizations, Mortality in Functional MR Patients With Severe HF
Physicians responded with shock and excitement to findings, but struggled to reconcile the results with the MITRA-FR trial.
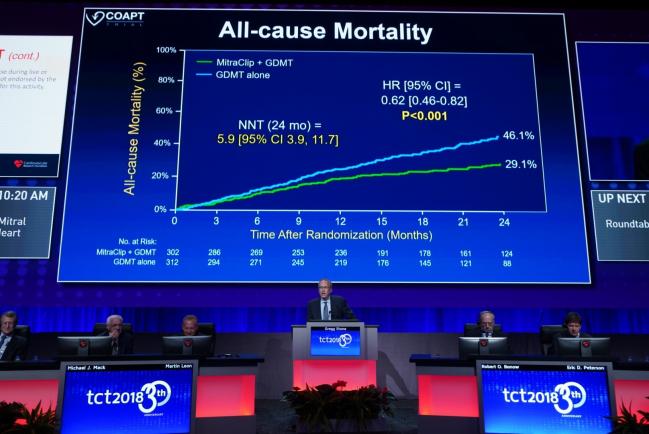
SAN DIEGO, CA (UPDATED)—It’s a shocking success for the MitraClip after years of gloomy predictions, sluggish trial enrollment, and—most of all—the negative MITRA-FR trial results last month. Today, the long-awaited COAPT results show that transcatheter mitral valve repair using the percutaneous clip procedure in patients with heart failure and severe functional mitral regurgitation (MR) significantly reduced not only the primary endpoint of heart failure (HF) rehospitalizations, but also mortality at 2 years.
The audience gave an audible gasp and broke into applause when the primary endpoint results, and later when the mortality results, went up on the big screen in the main arena.
“For patients, this is the dawn of a new day,” lead investigator Gregg W. Stone, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), told TCTMD. “For high-risk patients with heart failure secondary to mitral regurgitation of a severe degree, who have failed all guideline-recommended therapies at maximally tolerated doses, who have also had cardiac resynchronization therapy and revascularization, as appropriate, the MitraClip offers these patients a new ray of hope.”
The COAPT results were the source of endless speculation in the lead-up to TCT 2018, where Stone presented the results today. It was just last month, at the European Society of Cardiology Congress 2018, when MITRA-FR, the first of several trials studying the MitraClip in functional MR, came up empty-handed. As reported by TCTMD, MITRA-FR was a randomized comparison of MitraClip and optimal medical therapy in 304 patients, which found no differences in rehospitalizations or all-cause death at 1 year, the composite primary endpoint.
Stone, who presented COAPT as a late-breaking clinical trial here at TCT 2018, believes the vastly different results between the two trials likely relate to patient selection, medication changes during the trial, level of operator experience and the outcomes achieved, as well as duration of follow-up. Others, however, seem to be reeling from the results, unable to reconcile the two trials or, by contrast, seeing them as more alike than different. Several experts who spoke with TCTMD cautioned that interventionalists who do these procedures need to closely study the specific patient group enrolled in COAPT before taking this therapy into a broader group of patients, or better yet, wait for more trial results to come in.
Full COAPT results were also published online in the New England Journal of Medicine to coincide with Stone’s presentation.
COAPT Results
The MitraClip (Abbott) has been in use for over a decade in Europe and the United States on the basis of randomized trials conducted in patients with degenerative (primary) mitral regurgitation. International guidelines either discourage the use of the MitraClip for patients whose mitral regurgitation is the result of left ventricular dysfunction or emphasize the lack of evidence supporting device efficacy in this setting.
COAPT, like MITRA-FR, set out to explore the use of clip coaptation in patients with MR secondary to left ventricular dysfunction who remained symptomatic despite guideline-directed medical therapy. The trial has faced its share of hurdles, including a revision to the endpoint prompting an expansion of patient numbers, initially anticipated to be 400, which in turn prolonged trial enrollment. Ultimately, 610 patients with moderate (3+) or severe (4+) mitral regurgitation were enrolled and randomized equally to guideline-directed medical care or the same, intensive medical management plus MitraClip implantation—a process that took 8 years to complete. Today, all that effort appears to have paid off.
As Stone showed here, the primary efficacy endpoint—all hospitalizations over 24 months—was significantly lower in the MitraClip versus the medical care group. Annualized rates of hospitalizations were 35.8% versus 67.9%, respectively (P < 0.001), yielding a number needed to treat of 3.1. Results in per-protocol, intention-to-treat, and as-treated analyses were very similar to the overall findings. Freedom from device complications in the MitraClip group was 96.6%. Perhaps most strikingly, 2-year mortality, a powered, prespecified secondary endpoint, was significantly lower among MitraClip-treated patients: 29.1% versus 46.1% (HR 0.62; 95% CI 0.46-0.82), yielding a number needed to treat of 5.9.
In COAPT, death or rehospitalizations for heart failure, which was the primary endpoint in MITRA-FR (measured at 1 year in MITRA-FR and 2 years in COAPT), was 45.7% in the MitraClip group versus 67.9% in the medical therapy arm (HR 0.57; 95% CI 0.45-0.71).
Other secondary endpoints also yielded significant differences. Quality of life as measured by the KCCQ score, as well as 6-minute walk test, both at 12 months, increased significantly in MitraClip-treated patients, but held relatively steady in the medical group. NYHA class also improved over various time-points, with significant differences seen between medical therapy and MitraClip groups. Left ventricular end diastolic volume increased substantially in the medical therapy arm over the first 12 months, but declined in the MitraClip group. While all patients had class 3+ or 4+ MR at the study outset, this proportion had fallen to less than 1% by 24 months in the MitraClip group. By contrast, the proportion in these two categories remained above 50% in the medically treated patients.
“I do think that the benefit this provides to patients is quite dramatic and important,” Stone told TCTMD. “For the heart failure docs, they now need to look at their HF patients. And because most heart failure experts are maximally treating their patients with the optimal doses that the patients can tolerate of guideline-directed medication therapies, now they need to go back and think, wow, I have something else to offer my patients who have 3+ to 4+ MR. So, I do need to identify those patients and then refer them, because I can substantially improve the quality of their life, improve their survival, and keep them out of hospital.”
Debate Will Continue
Asked why the COAPT findings are so radically different from those of MITRA-FR, Stone pointed to the larger size of the trial and the experience of the operators, and he also suggested that that the degree of MR among patients enrolled in COAPT was substantially more severe than in the European trial. That’s reflected in a mean effective regurgitant orifice area (EROA) in COAPT of 41 mm2 and in MITRA-FR of 31 mm2. Conversely, left ventricular end diastolic volume in COAPT was actually smaller than that in the French trial: 101 mL/m2 versus 135 mL/m2.
Uptitration of heart failure medication after randomization in MITRA-FR may also have been a factor, said Stone, noting that MITRA-FR adjustments were made according to “real-world” practice, while in COAPT patients had to be absolutely maximally tolerated prior to enrollment. This was a possibility raised at the recent London Valves meeting, where MITRA-FR was extensively discussed. At the time, Jean-François Obadia, MD (Hôpital Cardiovasculaire Louis Pradel, Bron, France), dismissed this hypothesis saying that, in his view, major changes to drug treatment were avoided as much as possible and compliance to medical therapy was “absolutely no different” between groups.
Stone, however, believes that the very stringent enrollment in COAPT helped ensure that all patients were maxed out on meds before entering the trial. As he explained to TCTMD, investigators held weekly eligibility calls to review potential trial participants and if, after a review spanning 2-years’ worth of medical therapy, a patient was found not to be taking “absolutely maximal doses that they could tolerate” of all guideline-directed drugs, that patient would be turned away from the trial. “We rejected a lot of patients like that and a lot of them never came back to us and we had heard that they got better,” Stone said.
But that type of patient selection, in part, explains why COAPT took so long to enroll patients. MITRA-FR, by contrast, was designed as a pragmatic trial. Contacted for his reactions to COAPT, Obadia insisted that MITRA-FR “represents more what is ‘real life.’” He made this same point at London Valves, namely that MITRA-FR represents precisely the types of patients receiving the MitraClip now.
“Today a large proportion of the indications probably do not add any benefit for the patient and therefore they should be avoided,” Obadia told TCTMD in an email. “Conversely, COAPT represents a highly selected population. In COAPT, the [highest] enrolling center, Cedars-Sinai in Los Angeles, included 46 patients during an enrollment period of close to 5 years. That is to say, less than one clip per month in the most active center in the COAPT trial. A very selected population!”
Stone himself agreed with this characterization of COAPT.
“I think it was highly selected,” he told TCTMD. “We wanted patients who were ill, who had substantial left ventricular dysfunction, but not too severe where their prognosis was going to be dominated by the amount of muscle damage as opposed to the contribution of the MR. Similarly, we wanted patients with truly moderate-to-severe or severe MR, and that had to be confirmed by the echocardiographic core lab before enrollment. So meeting the criteria of being symptomatic despite failing guideline-directed medical therapy at maximal doses was a signature of the trial and yes, while that did increase the selectivity of the trial and prolong its duration of enrollment, it led us to define the population in whom the MitraClip is going to have marked benefits. The clinical implication is that we would not recommend that people get treated with the MitraClip for severe MR until they have truly failed all maximally tolerated doses of guideline-recommended therapy and [cardiac resynchronization therapy] if appropriate.”
Indeed, Obadia believes the results are not so much contradictory as complementary: “There is absolutely no contradiction between their results,” he told TCTMD. “Thanks to these two studies we know with MITRA-FR what should be avoided and with COAPT what should be the ideal target for the heart team when we discuss an indication for the MitraClip.”
Rolling Out in the Real World
During a morning press conference, a range of physicians expressed their surprise and excitement about the results.
“I would really characterize this, to use a lay term, as a blockbuster trial because you see a statistically significant reduction in cardiovascular mortality, which is something you almost never see in device-based cardiovascular trials,” said Martin Leon, MD (NewYork-Presbyterian/Columbia University Irving Medical Center). “It is unusual in that regard. At the same time, you hit your hard primary endpoint with a number needed to treat of less than 10, and in this case, low single digits. So, from that standpoint I think this is a landmark trial that is going to change clinical practice.”
Jeffrey Popma, MD (Beth Israel Deaconess Medical Center, Boston, MA), likewise, called the trial “breathtaking.”
“I looked at these results and the first time I saw them, it just knocks you off your chair,” Popma said. “This was very difficult to perform, but the results are just spectacular.”
It just knocks you off your chair. This was very difficult to perform, but the results are just spectacular. Jeffrey Popma
Co-principal investigator Michael Mack, MD (The Heart Hospital Baylor, Plano, TX), agreed: “This is the most difficult trial I have ever been involved with, and it’s also the most positive trial I’ve ever been involved with. This is seminal in this field.”
Experts seemed to agree that it was the quality and rigor of the trial that led to the surprisingly positive results, and precisely the same trial characteristics that will make it difficult to replicate in the real world. Mayra Guerrero, MD (Mayo Clinic, Rochester, MN), cautioned that operators should not “adopt this approach for everybody” or make the decision to implant a clip without consultation with a heart team, which should now include a heart failure expert.
Mack, likewise, stressed that in addition to the skill of the operators in COAPT, the degree of guideline-directed medical therapy cannot be overemphasized. “That may be one of the key reasons why this trial was so positive. It’s well known in the field of heart failure that device therapy is more effective when [the patient] is on optimal, guideline-directed medical therapy, so the message to the community is going to be: if MitraClip is going to be effective, these patients need to be treated with [guideline-directed medical therapy] and that’s another generalizable aspect of this.”
Stone himself agreed. “Interventional cardiology as a subspecialty has evolved a lot over the last 40 years since its introduction by Grüntzig, from ‘Can we do it?’ to ‘Should we do it?’ And I think that applies to the MitraClip just as it does to percutaneous coronary interventions,” he stressed. “The MitraClip, in experienced hands, is a very safe procedure with a very high success rate, but now we have to identify the patients who should be treated and not who can be treated. And for the interventionalists, there is an opportunity and a caveat. We now obviously want to offer this therapy to the appropriate HF patients who have the right anatomy to be able to reduce secondary mitral regurgitation, the right degree of LV dysfunction and dilatation, etc, but they must have failed medical therapy at optimal guideline-directed doses.”
Understanding the Physiology
Others who spoke with TCTMD seemed both buoyed and genuinely flummoxed by the data, including Sanjay Kaul, MD (Cedars-Sinai Medical Center, Los Angeles, CA), whose center—as Obadia noted—was the number-one enroller in COAPT. Kaul said he himself saw, firsthand, the phenomenal improvements in patients and agreed that a placebo effect could not account for the mortality benefits, but still finds the results difficult to reconcile with MITRA-FR.
“I describe myself as a forensic evaluator of evidence,” he told TCTMD, “and I cannot poke any holes in the COAPT trial because the echo, the clinical, the functional, the health status outcomes are all coherent, they are all aligned, and so that’s why I’m having difficulty explaining it: Why is there a positive result?”
Part of what Kaul was grappling with were the explanations for the differences between the two trials. The suggestion that COAPT included a higher-risk cohort is challenged by the fact that mortality rates were relatively similar in the control arms of both trials, he pointed out. Kaul was also uncomfortable with the idea that residual MR could explain the different outcomes: 17% in MITRA-FR and 5% in COAPT. “That assumes that there is a very steep relationship between residual MR and 1- or 2-year mortality and that is open to question,” said Kaul.
If you have one trial that is null and you have another trial that is profoundly positive, how do you decide which trial is credible or reliable? Sanjay Kaul
“The bottom line is, I do not find the differences of sufficient magnitude to account for the discordant results, and as such, a reasonable conclusion—unless there are some other cogent explanations that escape me—the reasonable conclusion is that we need additional data to resolve this uncertainty,” Kaul said. “And if those are positive, then I will be the first one to say, hey, let’s take the trumpet out and celebrate. Because this is a historic milestone, this is unexpected and unprecedented. But why would fixing a valve in a diseased myocardium impact mortality related to diseased myocardium? That’s a fundamental pathophysiologic question.”
The pathophysiology came up during COAPT’s presentation today, with several experts suggesting that the trial may turn the understanding of disease progression on its head.
“We’ve long felt that this disease, heart failure and mitral regurgitation, is a combined disease and it’s been a chicken or an egg problem, whether it is caused by the MR or the left ventricle,” Howard Herrmann, MD (Penn Heart and Vascular Center, Philadelphia, PA), said during the press conference. “In general we’ve been reluctant to operate on these patients because we felt that the ventricle was more of a problem than the MR. Your data would suggest that that’s not entirely true. And in fact, if you look at your control group, the secondary endpoints tended to get worse over time, whereas in the MitraClip group they tended to stabilize, suggesting that the MR has a pathophysiologic role in the degeneration of the ventricle.”
Stone agreed, noting: “What I think we’ve seen here is that secondary MR is not just a bystander. Many heart failure experts thought this was a marker of a sicker left ventricle, but we’ve seen now that it does contribute to the abnormal pathophysiology of these patients. We didn’t cure patients by fixing their mitral regurgitation—we still have 29% 2-year mortality—but we did markedly improve their quality of life, their readmissions for HF, and their survival, somewhat, by at least interrupting that part of the abnormal pathophysiology.”
This observation, predicted Ted Feldman, MD (Evanston Hospital), will have an impact far beyond the MitraClip. “This trial result will define the next wave of development in mitral therapies,” he told TCTMD. “The fundamental differences between replacement devices and repair devices is the surety with which MR is obliterated with replacement. And the question now is, will even greater reduction in MR leave to incremental improvements over what we just saw in COAPT? And that’s left to be answered.”
Heart failure expert Mary Norine Walsh, MD (St. Vincent Heart Center, Indianapolis, IN), a site investigator for COAPT, who spoke with TCTMD today, called the results “exciting” and “a surprise.” That surprise stems from the very different results between COAPT and MITRA-FR, where she, like Kaul, did not see major differences in terms of the patients enrolled.
She also stressed the need for heart failure specialists to be the gatekeepers, emphasizing that the inclusion of HF doctors was a unique aspect of COAPT. “I think the proviso is, please get the patients to us so we can decide what therapy is best, because sometimes medical therapy is excellent.” Indeed, one detail that should still be looked into is the use of the newest heart failure drug, sacubitril/valsartan (Entresto; Novartis) during the trial. “Mixed into COAPT enrollment came valsartan-sacubitril, and I don’t think it’s been reported how many patients were on that,” Walsh said.
What Happens Next
Whether the US Food and Drug Administration (FDA) will be persuaded by the COAPT data to expand current device indications to embrace secondary MR remains to be seen. Stone seemed confident that the powerful COAPT data speaks for itself. “I believe the FDA should act on these data. I don’t speak for what the FDA should do, but we've been in communication,” he said. Investigators have also dug into the data to help the FDA target this therapy and have identified the only subgroup who did not benefit in COAPT, namely, the 10% of patients who had an EROA of < 30 mm2 and a left ventricular end diastolic volume greater than the median.
Kaul, however, stressed that he hopes the FDA will wait for RESHAPE HF 2, which is rumored to be nearing completion.
“If you have one trial that is null and you have another trial that is profoundly positive, how do you decide which trial is credible or reliable?” Kaul asked. “And the long and the short answer is that logic and common sense would dictate that you need additional data to resolve the uncertainty. You need a tiebreaker, another trial. If the results of the third trial are consistent with COAPT, then I think one could say with some degree of certainty that the COAPT results indeed credible and reliable enough to impact regulatory and clinical decision-making. That to me is objective, common sense perspective. I have no skin in the game, I’m an outsider looking in at the evidence.”
Kaul also stressed that for interventional cardiologists to take COAPT as a greenlight to ramp up use of the MitraClip “would be a mistake.” Moreover, “it would be a mistake for the FDA to expand the indication purely on the basis of this. What if it turns out this was wrong? As a responsible scientist, keeping the societal perspective and the patient in mind, I think it is imperative that the regulatory agencies wait and collect more information before they act on it.”
Walsh, for her part, reiterated that the results are “pretty exciting.”
“Even with aggressive medical therapy there are patients who have this degree of MR and I would anticipate that there will be much wider use of the MitraClip in these patients, and that may help some to avoid [ventricular assist devices] and transplants,” Walsh said.
During the press conference, Stone estimated that approximately 10% of heart failure patients likely match the profile of patients treated in COAPT; Walsh agreed with that number.
Like Kaul, however, Walsh would love to see one more trial add some clarity to explain the very different results that have rocked this space in the last month. “I believe these results, but what gives me pause is the difference between MITRA-FR and COAPT. I’m not sure the question is fully answered because both studies were done very well.”
RESHAPE HF 2 investigators now have some soul-searching to do. Stefan Anker, PhD (Charité University, Berlin, Germany), one of the principle investigators for RESHAPE HF 2, was one of the discussants following Stone’s presentation today. He pointed out while the highest-enrolling center in COAPT implanted, on average, one clip per month, interventional cardiologists in some parts of Europe are placing between two and five clips per week. On the flip side, mortality among these patients is not as high as the mortality seen in either MITRA-FR or COAPT.
“I can only ask the community to further support research because the results also document that if you go for milder patients, 650 patients will not be enough,” Anker said. “Our trial is currently planned at 420 patients; you will of course understand that this will now be an interesting discussion with the [data safety and monitoring board], how to resize this study.”
During today’s session, Leon asked Robert Bonow, MD (Northwestern University, Chicago, IL), about the potential impact of COAPT on valve guidelines. “Bob, is there no way we can sneak this in as class I recommendation, even if we include the heart failure people as partners?”
“There’s no doubt will cause some ruffles in the guideline committee and of course we’ll have to reconvene quickly to assimilate this information,” Bonow replied. “But we do have another trial on the way with RESHAPE HF 2, and that has to be balanced as well with MITRA-FR. We now have manuscripts we can begin to dissect, and we can go through all the supplemental data as well. We’ll have to see if we agree with Gregg’s analysis as to how they differ, and can we identify subsets of patients where this might move up or do we need a higher level of evidence? I’m going to hold my cards for now.”
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Stone, GW, Lindenfeld JA, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;Epub ahead of print.
Disclosures
- Stone disclosed a research grant from Abbott paid to the Cardiovascular Research Foundation, as well as personal fees from Claret, Ablative Solutions, Mitrizyme, Miracor, Neovasc, V-wave, Shockwave, Valfix, TherOx, Reva, Vascular Dynamics, Robocath, HeartFlow, and Gore, and equity/options from MedFocus family of funds, Ancora, Cagent, Qool Therapeutics, and Aria.


Comments