IMPROVE-HCM: Ninerafaxstat May Fill a Niche in Nonobstructive HCM
With a drug targeting the heart’s energy efficiency, the phase II trial showed better exercise capacity and fewer symptoms.
ATLANTA, GA—Ninerafaxstat (Imbria Pharmaceuticals), a novel drug targeted at patients with symptomatic nonobstructive hypertrophic cardiomyopathy (HCM), is safe and well tolerated, according to data from the IMPROVE-HCM phase II trial. Exercise performance improved over 12 weeks as well, and in the patients most limited at baseline, the drug led to gains in health status.
“There’s been a lot of advances in the treatment of obstructive HCM over the last several years and decades,” said IMPROVE-HCM investigator Martin S. Maron, MD (Lahey Hospital and Medical Center, Burlington, MA), presenting results from the randomized study at the recent American College of Cardiology (ACC) 2024 Scientific Session. The data were simultaneously published online in the Journal of the American College of Cardiology to coincide with the meeting.
For obstructive HCM, the US Food and Drug Administration approved mavacamten (Camzyos; Bristol Myers Squibb), an allosteric inhibitor of cardiac myosin, in early 2022 based on results from the EXPLORER-HCM trial. Top-line results for another drug of the same class, aficamten (Cytokinetics), from the phase III SEQUOIA-HCM trial also are encouraging in that population.
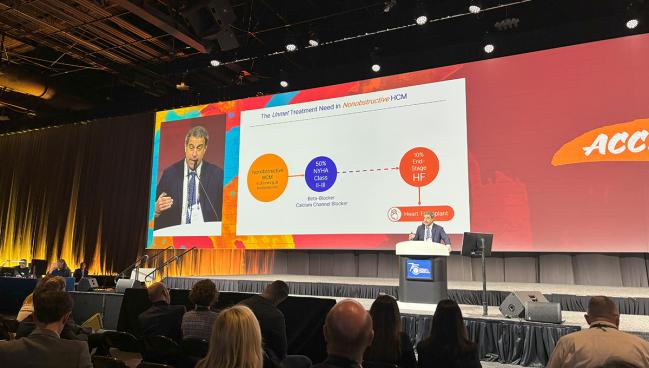
To date, there have been no targeted therapies approved for nonobstructive HCM, Maron said. “The unmet need here is to develop additional therapies that can relieve this substantial burden of symptoms and improve quality of life, and also to potentially mitigate progression.”
About half of patients with the disease are in NYHA class II or III despite taking beta-blockers and calcium channel blockers, he told the ACC audience. A subset of about 10% progresses to end-stage heart failure.
“What’s driving heart failure in nonobstructive HCM is diastolic dysfunction,” which occurs due to numerous factors related to LV relaxation and distensibility: hypertrophy, ischemia, disarray, interstitial fibrosis, and replacement scar, Maron explained. “Importantly, [this] process is very heavily energy dependent.”
Ninerafaxstat, a cardiac mitotrope, plays off this latter characteristic. It helps the myocardium work more effectively by partially inhibiting fatty acid oxidation, thus encouraging the use of glucose as fuel. “It does not require any drug monitoring, and has no hemodynamic effect,” he said.
Clyde Yancy, MD (Northwestern University Feinberg School of Medicine, Chicago, IL), the discussant for IMPROVE-HCM, agreed that the trial addresses a “critically important but unmet need” in focusing on the nonobstructive phenotype of the disease.
“We typically see patients, perhaps predominantly so, who don’t have an obstructive gradient and are not candidates for the new class of antimyosin therapies,” he commented. “Yet they’re very symptomatic. They come to the office discouraged and leave the office discouraged, treated only with very-high-dose beta-blockers, from which they realize even more side effects. So I really do think this is very necessary work.”
It does make sense, Yancy said, to target energy efficiency in this setting.
For EXPLORER-HCM investigator Florian Rader, MD (Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA), the results on ninerafaxstat are encouraging but not definitive. “For nonobstructive HCM, there are just no well-studied or FDA-approved medications whatsoever,” he told TCTMD. “There’s really no good option right now, so I’m happy to see that at least there’s something else potentially in development.”
Nonobstructive and obstructive HCM, mechanistically speaking, are “on two different, parallel tracks,” Rader explained. That said, one thing the two forms have in common is that in both, the heart pumps harder than it should. Both mavacamten and aficamten, which can reduce this hyperdynamic contraction, “are being studied in large phase III trials for nonobstructive HCM right now,” he noted, but “we have to wait and see.”
Rader, too, agreed the ninerafaxstat merits further study. “Maybe this is a candidate, but I think we need more data before we can be sure that this is beneficial,” he added.
IMPROVE-HCM
Across 12 centers, Maron et al enrolled 67 patients (mean age 57 years; 55% female) with nonobstructive HCM plus LV outflow gradient < 30 mm Hg, LVEF ≥ 50%, and predicted peak VO2 < 80%. At baseline, the mean peak VO2 was approximately 19 mL/kg/min, amounting to 60% of the predicted peak VO2 based on age and gender. About 60% were in NYHA class II and 35% NYHA class III. Nearly six in 10 patients (58%) were already on beta-blockers and 32% on calcium channel blockers.
Study participants were randomly assigned to receive ninerafaxstat 200 mg twice daily or placebo for 12 weeks.
In the ninerafaxtat group, four patients (11.8%) experienced treatment-emergent serious adverse events: diverticulitis, pyelonephritis, CABG, and COVID-19 pneumonia. By comparison, two patients (6.1%) in the placebo group had a severe adverse event: septic shock and acute hypoxia.
Less severe treatment-emergent events occurred in 70.6% of the treatment group and 60.6% of the placebo group, though most were “mild to moderate,” said Maron. The most common, experienced by more than 4% of patients, were ventricular tachycardia, nausea, headache, hypokalemia, palpitations, diarrhea, chest discomfort, and fatigue—in particular, the number of patients who had nausea, headache, hypokalemia, chest discomfort, and fatigue trended higher with ninerafaxstat compared with placebo. There were no changes in LVEF, blood pressure, or heart rate.
Between baseline and 12 weeks, patients randomized to ninerafaxstat grew to have better ventilatory efficiency (VE/VCO2 slope) than those receiving placebo, with a least square mean difference of -2.1 (P = 0.006), but no difference in peak VO2 (P = 0.9). Given that worsening ventilatory efficiency is linked to future risks of death and transplant in HCM, “I think it’s reasonable to conclude that that’s a clinically meaningful change in exercise capacity with treatment,” Maron said.
Changes in Kansas City Cardiomyopathy Questionnaire-Clinical Summary Score (KCCQ-CCS) over the 12 weeks were similar, on the whole, with ninerafaxstat and placebo (least square mean difference -3.2; P = 0.2). But in patients who started out at KCCQ-CCS ≤ 80, there was a significant gain in health status (least square mean difference 9.4; P= 0.04), as was the case for patients who started out in NYHA class III (least square mean difference 13.6; P = 0.03).
By week 12, left atrial size had decreased with ninerafaxtat (mean -0.9 mm vs baseline) but not with placebo (mean 0.10 mm vs baseline; least square mean difference -0.20; P = 0.01). Maron said this likely reflects “the effects of ninerafaxtat on improving diastolic function, which resulted in favorable remodeling in left atrial size.”
Maron said the strength of their IMPROVE-HCM findings suggests it’s worthwhile to continue now with a phase III trial.
While IMPROVE-HCM does confirm ninerafaxtat is safe and well tolerated, “when it comes to effectiveness, I think it is a little disappointing,” Rader said.
For instance, although ventilatory efficiency improved, this is not the usual outcome the FDA would use when deciding on whether to approve the drug, he said. Moreover, NT-proBNP levels did not differ between the medication and placebo. The heart failure marker is “usually very sensitive to changes in intracardiac pressure, which correlate very closely to how patients feel,” Rader explained. The lack of difference “makes me a little bit worried that this medication doesn’t work quite as well as we think.”
The IMPROVE-HCM researchers have a theory about the lack of impact on NT-proBNP. “Based on the mechanism of release of natriuretic peptides related to myocardial wall stress, it is plausible that longer treatment periods may be necessary to achieve reverse cardiac remodeling to enhance diastolic function in order to observe a significant effect on NT-proBNP values,” they write.
Himabindu Vidula, MD (Penn Medicine, Philadelphia, PA), at an ACC press conference, commented that the early results seen with ninerafaxtat strongly argue for further study. “All of these changes occurred over a relatively short period of time of only 12 weeks of treatment, and this really underscores the potential for enhancing the outcomes with a longer exposure time to the drug,” she said.
Yancy, during the session’s discussion, asked Maron whether the benefits seen with ninerafaxtat might eventually mean that high-dose calcium channel blockers and beta-blockers were unnecessary, “which would be a significant relief for patients.”
“Certainly, it’s possible that if we can ultimately show efficacy in the two important endpoints here that matter most, . . . feel and function, [then] a drug like this could be first line or replace the need for calcium channel blocker or beta-blocker therapy,” Maron said. “I think it’s an achievable goal.”
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Maron MS, Mahmod M, Samat AHA, et al. Safety and efficacy of metabolic modulation with ninerafaxstat in nonobstructive hypertrophic cardiomyopathy: phase 2 study. J Am Coll Cardiol. 2024;Epub ahead of print.
Disclosures
- Imbria Pharmaceuticals contributed significant funding to this study.
- Maron reports being on the steering committee for SEQUOIA-HCM and receiving consultant/advisor fees from Cytokinetics, Imbria, and Edgewise.
- Rader is a consultant for Cytokinetics and Bristol Myers Squibb.






Comments