Bioprosthetic Valve Thrombosis Rare but Not Benign: New Insights
Surgical valve thrombosis is often missed yet carries important risks. Experts offer some tips for treatment and follow-up.
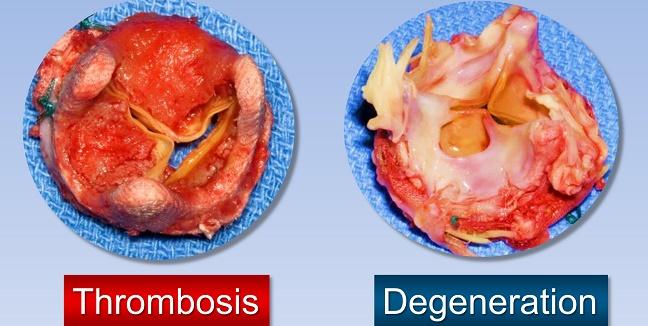
Though rare, bioprosthetic valve thrombosis (BPVT) is associated with both a recurrence of BPVT and early prosthetic degeneration, according to a new analysis. Treatment with anticoagulation seems to improve the long-term outcomes of patients with BPVT, but it can cause an increase in bleeding.
“The important message is to keep close tabs on these patients and consider anticoagulation unless they have high risk of bleeding,” senior author Sorin Pislaru, MD, PhD (Mayo Clinic, Rochester, MN), told TCTMD.
For the study, published in the March 3, 2020, issue of the Journal of the American College of Cardiology, Ioana Petrescu, MD (Mayo Clinic), Pislaru, and colleagues matched 83 patients treated with warfarin for suspected BPVT at their institution between 1999 and 2017 with 166 controls. The largest proportion of valves were placed in the aorta (48%), followed by the tricuspid (25%), mitral (22%), and pulmonary (5%) positions, and only 8.4% were transcatheter devices. While three-quarters of the treated group had normalized echocardiography within 3 months, the remaining 25% did not respond to warfarin.
Over a median follow-up of 34 months, the researchers found no difference in the primary composite endpoint (P = 0.79)—stroke, peripheral embolic events, or all-cause death—or mortality alone (P = 0.73) between the patients with BPVT and matched controls. However, major bleeding was more common in the BPVT group (12% vs 2%; P < 0.0001).
BPVT recurred in 23% of warfarin responders within a median 23 months, with a hypercoagulable state identified in four of the 14 patients who had a second event. Two patients had a third episode of BPVT.
Patients with BPVT also had a higher probability of repeat valve replacement at 10 years (68% vs 24%; P < 0.001).
‘What Happens Long-term?’
Before this study, it was commonly felt that if one episode of BPVT is successfully treated, “you cure the disease . . . because the way the valve looks like on echo, on CT, [is] completely normal,” Pislaru said. “But the question is: what happens long-term? Did you solve the issue for good? Do these patients have the same longevity of the prosthesis or not? And number two, do they get a second episode?”
These study findings confirm the need for more aggressive follow-up, he stressed, noting that his institution follows and images this population on a yearly basis. Additionally, what “was quite surprising to all of us was that in the patients who had an episode of thrombosis even if you treat them well—the ones that had success with warfarin—their bioprosthesis fails earlier than controls. So, it means that something is permanently damaged, at least in a number of these patients.”
The second implication for practice, according to Pislaru, is that patients with an “an acceptable risk of bleeding” should remain on chronic anticoagulation. “Because if you say one in four patients have a second episode, [they] might as well stay on chronic anticoagulation unless there's a significant risk of having a bleed,” he said. For those with bleeding complications, a second valve-in-valve procedure might be their only option.
Every center should develop a practicable algorithm with different imaging modalities and specialists to ensure the correct diagnosis of BPVT. Kerstin Piayda
Commenting on the study for TCTMD, Kerstin Piayda, MD (Heinrich Heine University Düsseldorf, Germany), agreed that the study findings should encourage physicians to keep patients with a BPVT episode on indefinite warfarin therapy if there are no contraindications. “Those patients still have an increased risk for valve replacement and might need a closer follow-up to determine the optimal time-frame,” she said in an email.
Additionally, Piayda said, “awareness for suspected BPVT is high and might lead to a significant number of misdiagnosed patients. Hence, every center should develop a practicable algorithm with different imaging modalities and specialists to ensure the correct diagnosis of BPVT. If a patient is put under warfarin for BPVT, close monitoring might be essential to ensure the optimal therapeutic range, which seems to be the key for an effective therapy.”
Implications for TAVR
In an accompanying editorial, Blase Carabello, MD (East Carolina University, Greenville, NC), notes that though few TAVR valves were included in the study, the findings have important implications for those devices.
“Thrombosis of TAVR valves occurs more frequently and earlier than it does in SAVR biologic valves,” he writes. “This finding might be due to crimping of the TAVR before deployment, or because the dead space left between the native valve and the TAVR leaflets is thrombogenic, or for other reasons not yet understood. However, three aspects of the current study seem likely to extend to TAVR and are worrisome: 1) the chance of recurrence; 2) the fact that BPVT, when it occurs, seems to make valve deterioration more likely; and 3) misdiagnosis of BPVT carries the risk of warfarin-induced bleeding without any benefit to the patient.”
Piayda also stated that these findings “might not be applied to all bioprosthetic valves if we take aortic valve replacement as a representative example.” For SAVR, she said, “valve thrombosis occurs less frequently and after a longer duration if compared to TAVR. The understanding of underlying pathomechanisms is [sparse], but valve crimping, thinner leaflet-thickness, and different hemodynamic conditions through design differences might be an issue.”
Pislaru said that given the limited number of TAVR patients in the analysis, it’s “definitely hard to extrapolate the conclusions, but I do not think that TAVR valves are different than surgically implanted valves. [If] you think about it, it's the same manufacturers, they make the valves out of the same materials, the only difference is the deployment type.”
Prior analyses have estimated the rate of TAVR valve thrombosis to be around 10%, he said. “I do not know if the number is as high as that. In our experience, it probably is going to be lower. But I am convinced that a number of patients with TAVR do have the same problems. Maybe one of the reasons we have so very few TAVR thromboses is because we changed our practice almost 5 years ago [to use] warfarin for the first 3 months in all patients unless they have a strong contraindication.”
When you start looking, you start seeing. Sorin Pislaru
For Carabello, “the goal is simple enough: to prevent BPVT without increasing the risk of dangerous bleeding, stroke, or vascular complications.” In order to do this, he recommends focusing on high-benefit/low-risk therapies; however, Carabello adds, “the current situation is fraught because it entails the relatively low but definite risk of BPVT versus the unknown risk and benefit of preventing it.”
DAPT with clopidogrel and aspirin for 6 months is current practice, but “this recommendation is entirely empirical,” Carabello writes. “Its efficacy is untested by randomized trials, and it is unclear what, if anything, is effectively treated by DAPT therapy. Furthermore, BPVT is only one consideration in planning a TAVR antithrombotic strategy. Stroke and vascular complications may also be affected. Stroke in TAVR is declining and in fact seems lower than with SAVR.”
After SAVR, many centers as well as guidelines recommend VKAs for 3 months, and it’s possible this could be a good option for TAVR patients, he says. “Preliminary data suggest that the single antiplatelet therapy may be as safe as DAPT treatment. One trial of a non-VKA anticoagulant (rivaroxaban) was stopped early because of excess complications. However, BPVT was reduced in the rivaroxaban group. Whether those results pertain to other non-VKA agents is unknown.”
Pending the results of ongoing trials examining a variety of antiplatelet and anticoagulant strategies, Carabello concludes that “it is likely that we will do the following: 1) use a short-term anticoagulant strategy together with or instead of DAPT therapy for TAVR; 2) routinely confirm suspected BPVT with computed tomography scan assessment of hypoattenuation leaflet thickening; and 3) use prolonged anticoagulation if BPVT does occur.”
Further Research Needed
In the future, Pislaru said he would like to see studies conducted “to understand more exactly the link between bioprosthetic thrombosis and degeneration, number one, and number two, why exactly do some people develop this and some people don't. . . . Hypercoagulable state clearly plays a role, but that's no surprise to anybody. Atrial fibrillation seems to play a role, but again is this the atrial fibrillation per se or is it whatever leads to atrial fibrillation? It's very hard to make the clear link between what's causing this and the risk factors we usually look at.”
Piayda also endorsed the need for larger trials and registry analyses assessing BPVT and subclinical leaflet thrombosis, with longer follow-up. “With increasing numbers, we should be able to separately investigate surgical and catheter-based valve replacement in each position (ie aortic, mitral, tricuspid, and pulmonary) and determine an optimal strategy to diagnose and treat (sub)clinical leaflet thrombosis,” she suggested.
Another issue with studying this complication is that it is often missed, Pislaru said. “There was no such disease until there was a disease, much like there was no pacemaker-induced tricuspid regurgitation until there was. . . . When you start looking, you start seeing.”
Photo credit: Sorin Pislaru
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Petrescu I, Egbe AC, Ionescu F, et al. Long-term outcomes of anticoagulation for bioprosthetic valve thrombosis. J Am Coll Cardiol. 2020;75:857-866.
Carabello BA. Bioprosthetic valve leaflet thrombosis: how the past may inform us about the future. J Am Coll Cardiol. 2020;75:867-869.
Disclosures
- Petrescu, Pislaru, Piayda, and Carabello report no relevant conflicts of interest.


Comments