BIOSTEMI: Degradable-Polymer, Ultrathin-Strut DES Shows No Late Catch-up at 5 Years
Two years after primary PCI for STEMI, Orsiro’s polymer is gone, making it essentially a BMS. But outcomes didn’t suffer.

SAN FRANCISCO, CA—The Orsiro biodegradable-polymer, ultrathin-strut, sirolimus-eluting stent (Biotronik) continues to show superiority over the Xience Prime/Xpedition durable-polymer, everolimus-eluting stent (Abbott Vascular) even 5 years after primary PCI for STEMI, data from the BIOSTEMI Extended Survival (ES) study show.
That advantage mainly comes from less ischemia-driven target lesion revascularization within the first 2 years after treatment.
BIOSTEMI ES, presented this week at TCT 2023 and simultaneously published in the Lancet, is an offshoot of the main BIOSTEMI randomized trial. At both 1 year and 2 years, Orsiro came out ahead in BIOSTEMI—the question in many people’s minds was what would happen after the 2-year mark, when the device’s biodegradable polymer had fully dissolved.
Juan F. Iglesias, MD (Geneva University Hospitals, Switzerland), lead author of the new paper, said the BIOSTEMI ES results demonstrate the absence of a late catch-up phenomenon. “I think these data are important for one reason: there’s still the question of what happens when the polymer degrades and we go back to a BMS-like stent platform,” he told the audience at the late-breaking session.
“What our study adds,” Iglesias said in a TCT press conference, “is the landmark analysis showing that the effects emerge within the first years when the polymer is in place,” suggesting the totality of Orsiro is what’s responsible, not a single characteristic. Moreover, there’s a plastic coating that stays on the platform even after the polymer is gone, he added. “This may probably explain why we don’t have this late catch-up phenomenon and increased risk of late events with this stent.”
Orsiro has long been on the market, having garnered CE Mark approval back in 2011 and then US Food and Drug Administration approval in 2019 based on the BIOFLOW V trial, which excluded STEMI patients. BIOFLOW V showed sustained advantages for Orsiro over Xience, specifically for target-vessel MI and stent thrombosis, but by 5 years the primary endpoint of target lesion failure was no longer significantly different between the two devices.
The potential mechanistic explanations for the observed differences at 5 years are a matter of speculation, to be honest: probably a combination of all individual components of the stent. Juan F. Iglesias
BIOSTEMI ES included 1,300 STEMI patients (n = 1,622 lesions) who had been randomized in the BIOSTEMI trial to receive Orsiro or Xience Prime/Xpedition over a 2-year period ending in March 2018. Complete 5-year follow-up was available for 85% of participants. The researchers used Bayesian statistical methods incorporating historical data on 407 STEMI patients enrolled in the 2014 BIOSCIENCE trial.
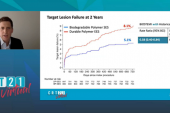
By 5 years, target lesion failure (cardiac death, target-vessel MI, or clinically indicated TLR) had occurred in 8% of the Orsiro group and 11% of the Xience Prime/Xpedition group (RR 0.70, 95% Bayesian credible interval [BCI] 0.51-0.95; Bayesian posterior probability for superiority 0.988). Most of the difference was driven by clinically indicated TLR, which occurred at rates of 3.1% and 5.4%, respectively (RR 0.68; 95% BCI 0.40-1.06; Bayesian posterior probability for superiority 0.956). Stent thrombosis rates were low and similar between groups, said Iglesias.
For both target lesion failure and TLR, the difference was restricted to the first 2 years post-PCI.
BIOSTEMI ES Landmark Analysis: Orsiro vs Xience Prime/Xpedition
|
|
RR |
95% BCI |
|
Target Lesion Failure 0-2 Years 2-5 Years |
0.57 0.87 |
0.39-0.83 0.46-1.52 |
|
Clinically Indicated TLR 0-2 Years 2-5 Years |
0.52 1.31 |
0.30-0.86 0.28-9.56 |
“The potential mechanistic explanations for the observed differences at 5 years are a matter of speculation, to be honest: probably a combination of all individual components of the stent,” said Iglesias. “So we have a metallic platform with quite thin struts; we have dedicated geometry; we have a biodegradable polymer, which is quite different from the others currently on the market because it degrades very slowly up to 2 years; and finally we have sirolimus at the low dose [with] a controlled kinetics release.”
Session moderator Ajay Kirtane, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), in discussion following Iglesias’ presentation, intimated that the polymer may be instrumental, as “the differences in strut thickness would probably only manifest in the smaller vessels, but yet you saw a consistent response.”
Now we have an ideal combination of platform, polymer, and drug release that improves clinical outcomes. Juan F. Iglesias
He then asked what he said might be a “provocative question.”
Clinical practice changed dramatically after the HORIZONS-AMI trial, “which was the study that essentially proved that DES reduced outcomes, predominately TLR, relative to a bare-metal stent,” said Kirtane. That 2008 trial “had an absolute risk reduction of 3% and this is a 5-year result but nonetheless most of it is seen in the first 2 years.”
Should the BIOSTEMI ES results, questioned Kirtane, now also be just as influential when it comes to stent choice?
“We have now a dedicated randomized clinical trial . . . in this subset of patients, so my answer would be yes,” Iglesias replied. “Now we have an ideal combination of platform, polymer, and drug release that improves clinical outcomes.” Repeat revascularization isn’t benign, as it can worsen quality of life, lead to procedural complications, and may even impair long-term survival, he added.
Robert Byrne, MBBCh, PhD (Mater Private Network, Dublin, Ireland), for his part, wanted to know more about why event curves separated as early as they did in the data.
“Unfortunately, I don’t think I have a definite explanation. There was no prespecified imaging substudy that would help with understanding the underlying process in terms of healing and neo-endothelialization. I think that it’s the combination of the whole stent design, which is highly biocompatible in this very highly prothrombotic and proinflammatory milieu, but we don’t have the answer to be honest,” said Iglesias.
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Iglesias JF, Roffi M, Losdat S, et al. Long-term outcomes with biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents in ST-segment elevation myocardial infarction: 5-year follow-up of the BIOSTEMI randomised superiority trial. Lancet. 2023;Epub ahead of print.
Disclosures
- The BIOSTEMI ES study was supported by a dedicated research grant to Inselspital, Bern University Hospital, University of Bern, from Biotronik.
- Iglesias reports a research grant to their institution, speaker fees, and support for attending meetings from Biotronik; research grants to their institution from Abbott Vascular, AstraZeneca, Biosensors, Concept Medical, Philips, and Terumo Corporation, outside the submitted work; and speaker fees from AstraZeneca, Biosensors, Biotronik, Bristol Myers Squibb, Cordis, Concept Medical, Medalliance, Medtronic, Novartis, Terumo Corporation, Pfizer, and Philips, outside the submitted work.




Comments