DAPT Study Shows Benefit of Long-Term Treatment After DES Implantation
CHICAGO, IL—In patients who receive drug-eluting stents, continuation of dual antiplatelet therapy (DAPT) beyond 1 year is associated with lower risks of stent thrombosis and major adverse cardiovascular and cerebrovascular events but a higher risk of bleeding, according to results of the long-awaited DAPT Study presented on November 16, 2014, at the American Heart Association Scientific Sessions and simultaneously published in the New England Journal of Medicine.
Laura Mauri, MD, of Brigham and Women's Hospital (Boston, MA), and colleagues designed the trial in response to a 2006 request by the US Food and Drug Administration (FDA) to manufacturers of coronary stents following a panel discussion. DAPT enrolled 25,682 patients within 72 hours of receiving an FDA-approved stent (89% DES) at 452 sites in 11 countries, and randomized 9,961 DES patients at 12 months to continue to receive aspirin plus a thienopyridine (50.4%) or aspirin plus placebo (49.6%) from August 13, 2009 to July 1, 2011.
Overall, one-quarter presented with AMI and more than half had at least 1 clinical or lesion-related risk factor for stent thrombosis. Mean patient age was 62 years and about one-quarter were women. Study drug discontinuation rates did not differ at 30 months between patients who continued DAPT and those who received placebo (21.4% vs 20.3%; P = .18). Clopidogrel was used in more patients than prasugrel (Effient; Eli Lilly; 65% vs 35%).
From 12 to 30 months post-implantation, rates of the primary efficacy endpoints of definite/probable stent thrombosis and MACCE (death, MI, or stroke) were lower in those who continued taking a thienopyridine than those who did not. Longer DAPT was also associated with a lower cumulative incidence of MI, with 55% of this benefit driven by a lower incidence of MI not related to stent thrombosis (HR 0.59). The study arms had similar rates of cardiac death, vascular death, and stroke, yet all-cause death was higher in those who continued thienopyridine treatment (table 1). Multivariable analysis confirmed these results.
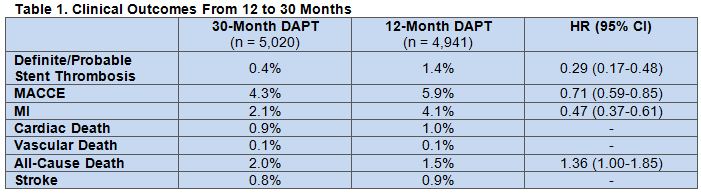
When the analysis was stretched out to 33 months (3 months after thienopyridine discontinuation in the long-term group), 30-month therapy still reduced stent thrombosis and MACCE compared with 12-month DAPT. Also, all-cause mortality was still higher in those who were on long-term treatment (HR 1.36). The authors attributed this difference to higher rates of fatal trauma- (P = .07) and cancer-related deaths (P = .02) in the thienopyridine group.
Because the rate of moderate to severe bleeding from 12 to 30 months was higher in those who received long-term DAPT vs placebo (2.5% vs 1.6%; HR 1.61; 95% CI 1.21-2.16), extended thienopyridine did not meet the criteria for noninferiority to 12-month treatment (P= .70). However, there were no differences between the treatment groups in terms of GUSTO severe or BARC fatal bleeding.
The effect of continued thienopyridine therapy vs placebo on the rates of the primary endpoints and MI was consistent across most subgroups.
FDA Replies
In an editorial accompanying the study, Antonio Colombo, MD, and Alaide Chieffo, MD, both of the San Raffaele Scientific Institute (Milan, Italy), cite the “recent drive within the interventional cardiology community to shorten the duration of dual antiplatelet therapy after implantation of a [DES] from 12 months… to 6 or even 3 months.” Because several trials and meta-analyses have shown shorter therapy to be safe in certain patients, they question whether the concept of extended therapy “may appear to be outdated.”
While DAPT shows ischemic benefits of continuing dual therapy past 1 year, the editorial notes, the concurrent increase in severe bleeding associated with long-term treatment “leaves us with uncertainty.”
Drs. Colombo and Chieffo say that the “key message of the DAPT Study is the suggestion that some patients who have been treated with a [DES] may benefit from extending dual antiplatelet therapy beyond 1 year, but also that the potential harm with this approach should not be overlooked. Moreover, we do not know how long this benefit extends and which patients benefit most.”
In a press conference, Mary Ross Southworth, PharmD, of the FDA, said the agency has only had the opportunity to do a preliminary review of the data and “has not reached a conclusion about risk [vs] benefit for prolonged dual antiplatelet therapy in patients receiving stents and cannot provide advice about how long to continue dual antiplatelet therapy following stent placement.”
For now, she continued, physicians should continue to prescribe clopidogrel and prasugrel as they have previously, and patients should continue their medications as prescribed.
‘We Have Enough’
In a discussion after the session, Gilles Montalescot, MD, PhD, of Pitié-Salpêtrière Hospital (Paris, France), commented that more than 35,000 patients have now been randomized in DAPT clinical trials over a period of 30 years. “I think we have enough to make up our minds,” he said. “We need to decide what is best for our patients.”
Still, some on the panel suggested a trial looking at even longer term DAPT might be necessary given that event curves somewhat diverge at the end of follow-up. Frans Van de Werf, MD, PhD, of University Hospitals Leuven (Leuven, Belgium), asked, “Should we ever stop dual antiplatelet therapy in this population?”
Additionally, “it seems to be that there might be a role for dual antiplatelet therapy for secondary prevention,” he said, adding that the PEGASUS trial “will hopefully confirm this message” when it is presented next year.
Robert A. Harrington, MD, of Stanford University Medical Center (Palo Alto, CA), said he is not going to change his practice based on this trial. If a patient has ischemic risk, he explained, “I’m going to have that conversation with you at 6 and 12 months and thereafter” about the risks of both continuing and stopping.
Ultimately, Dr. Mauri said, “personalizing these decisions is the holy grail.” In the future, she continued, treatment will be able to be better targeted, and “perhaps we [will better be able to] identify risk” with platelet biomarkers.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;Epub ahead of print.
Colombo A, Chieffo A. Dual antiplatelet therapy after drug-eluting stents—how long to treat [editorial]? N Engl J Med. 2014;Epub ahead of print.
Disclosures
- DAPT was supported by Abbott, Boston Scientific, Bristol-Myers Squibb-Sanofi, Cordis, Daiichi Sankyo, the Department of Health and Human Services, Eli Lilly, and Medtronic.
- Dr. Mauri reports relationships with multiple pharmaceutical and device companies.
- Drs. Chieffo and Colombo report no relevant conflicts of interest.


Comments