Dedicated Catheter for Acute PE Shows Promise in IDE Trial
The device requires RCT testing against other PE therapies and must show an advantage in hard outcomes, Marc Bonaca says.
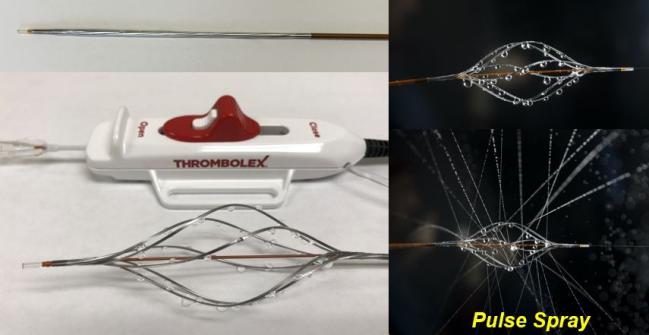
Photo Credit: Adapted from Bashir R. Prospective multicenter trial of pharmaco-mechanical catheter-directed thrombolysis with the Bashir endovascular catheter for acute pulmonary embolism: the RESCUE study. Presented at: TCT 2022. Boston, MA.
BOSTON, MA—Catheter-directed thrombolysis with a novel endovascular catheter that delivers low doses of recombinant tissue-type plasminogen activator (r-tPA) to the pulmonary arteries is effective in improving right ventricular-to-left-ventricular (RV/LV) diameter ratio in patients with acute pulmonary embolism (PE), with low rates of bleeding, early data show.
In recent years, catheter-directed thrombolysis and percutaneous mechanical thrombectomy devices have been a focus of efforts to improve outcomes of patients experiencing acute PE. In his presentation of the single-arm RESCUE trial here at TCT 2022, Riyaz Bashir, MD (Temple University Hospital, Philadelphia, PA), co-inventor of the new device, said it resulted in a mean change in RV/LV diameter ratio of 33% at 48 hours (primary endpoint) with a rate of major bleeding event of < 1%.
The study results were simultaneously published in JACC: Cardiovascular Interventions.
Speaking with TCTMD, Bashir said he created the device to improve upon existing catheter-based thrombolytic therapies that employ devices that are too small to function properly in pulmonary arteries. “We were putting this 2-mm catheter in these big vessels that are full of blood and expecting that they were going to open up that vessel and clean it,” he said. “I wanted to develop a device that would be dedicated for large vessels.”
The low-profile 7-Fr Bashir endovascular catheter (Thrombolex) consists of an expandable basket of six nitinol-reinforced limbs that each have multiple laser-drilled holes, for a total of 48 orifices that can disperse drug. Resembling a lawn sprinkler, it is advanced into the pulmonary arteries via femoral or internal jugular access and guided into the clot. The operator then expands the basket and releases a 1-mg spray of r-tPA diluted in 10 cc of normal saline and collapses the basket. This step is repeated to create fissures within the thrombus to enhance dispersion of the drug. In the final step, the basket is again expanded and a 5-mg infusion of r-tPA is given over 5 hours for a total of 7 mg of r-tPA per lung treated. The basket can expand up to 45 mm. After completion of the infusion, the devices can be removed at the patient’s bedside.
“I think this would be a very attractive as an option if they were able to show in a randomized trial that there were hard outcomes benefits,” Marc Bonaca, MD (University of Colorado School of Medicine, Aurora), who was not involved in the study, commented to TCTMD. He added that while other catheters have shown similar results for treatment of PE, the Bashir catheter needed a very low dose to dissolve the clot, which could be an advantage over others, as could the low rate of bleeding and device-related adverse events.
“It’s overall very encouraging,” Bonaca added. “But although these short-term surrogate outcomes correlate in natural history with bad outcomes, we don't really know whether that translates into somebody feeling better or living longer. To change practice, you need to show an impact on hard outcomes.”
RESCUE for PE
RESCUE enrolled 109 intermediate-risk acute PE patients (mean age 57 years; 61.5% male) from 18 centers in the United States. The vast majority of patients (n = 103) had bilateral PE. Approximately 14% had a prior history of PE and 26% had a prior history of DVT. Troponin and/or brain natriuretic peptide levels were elevated in 90%. Prior to the procedure, 93.6% received anticoagulation with heparin and 6.4% received enoxaparin sodium.
Patients with bilateral PE were scheduled to receive two devices, one in each pulmonary artery, while the remaining patients received just one device. The total dose of t-PA was 7 mg per device, so patients with bilateral PE received a total of 14 mg of r-tPA. Median device placement time was 15 minutes, total procedure time was 54 minutes, and the mean hospital stay was 2.88 days.
The procedure was successful in all patients. At 48 hours, the mean difference in RV/LV ratio was 0.56 ± 0.41 (P < 0.0001). There was also a 36% reduction in pulmonary artery obstructive index on CT, from 22.42 to 14.35 on the modified Miller score. One major bleed occurred within 72 hours in a patient who also had device-related left common iliac vein thrombosis while off anticoagulation. Four procedure-related safety events were seen in three patients through 30 days (epistaxis requiring silver nitrate cautery, non-access site hematoma, anemia, and retroperitoneal bleed). One patient died of non-PE-related causes within 30 days.
In the paper, Bashir and colleagues say the bleeding rates compare favorably with systemic thrombolysis, most contemporary catheter-directed therapies, and are lower than some contemporary mechanical thrombectomy studies.
“Ultimately, what is needed in the therapy of PE is to have a therapy that reduces blood clots as much as a systemic thrombolytic therapy would and does it safely, which is what the RESCUE trial showed,” Bashir told TCTMD.
In the discussion following his presentation, co-moderator Juan Granada, MD (Cardiovascular Research Foundation, New York, NY), said given that acute PE is a life-threatening condition, RCTs will be challenging to address and will need to be constructed appropriately in order to move toward regulatory approval of the novel device.
Bashir said he envisions an RCT of the device in comparison to oral anticoagulation in intermediate-risk patients and in comparison to systemic thrombolytic therapy in high-risk patients.
“This world of pharmacoinvasive strategies for acute pulmonary embolism is rapidly evolving,” Bonaca noted. “Where all of this is really going, hopefully, is toward risk stratification to target individual therapies to individual patients. At the end of the day, if you're not making people feel better and you're not preventing irreversible harm, it's hard to know whether some of these interventions are worth it because they cost money and there are risks.”
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Bashir R, Foster M, Iskander A, et al. Prospective multicenter trial of pharmacomechanical-catheter-directed thrombolysis with the Bashir endovascular catheter for acute pulmonary embolism._J Am Coll Cardiol Intv_. 2022;Epub ahead of print.
Disclosures
- Bashir is the co-inventor of the Bashir endovascular catheter and has equity interest in Thrombolex.




Comments