Endovascular Stroke Therapy Works for Large Infarcts: RESCUE-Japan LIMIT
In this Japanese study, the intervention improved 90-day functional outcomes, at the cost of an increase in ICH.
Patients with acute large-vessel occlusion strokes with large ischemic cores derive a benefit from endovascular therapy (EVT), the randomized RESCUE-Japan LIMIT trial shows.
“This is the first RCT showing the effectiveness of EVT for [strokes with a] large ischemic region,” Shinichi Yoshimura, MD, PhD (Hyogo College of Medicine, Nishinomiya, Japan), reported at the International Stroke Conference 2022.
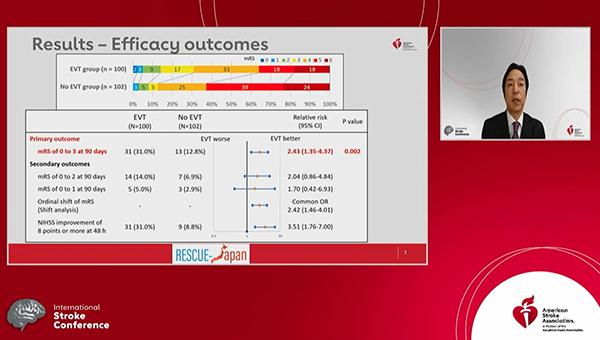
The findings, published simultaneously online in the New England Journal of Medicine, demonstrate that EVT plus medical therapy increased the proportion of patients with a favorable functional outcome—modified Rankin Scale (mRS) score of 0 to 3—at 90 days compared with medical therapy alone (31.0% vs 12.7%; relative risk 2.43; 95% CI 1.35-4.37).
That came at the cost of a higher rate of any intracranial hemorrhage (ICH) in the EVT arm (58.0% vs 31.4%; P < 0.001), although the difference in symptomatic ICH did not reach significance (9.0% vs 4.9%; P = 0.25).
We hope more patients with large infarctions will be saved by receiving EVT based on this trial. Shinichi Yoshimura
Overall, the trial “provides strong evidence that EVT improved patients when the infarction was large, ASPECTS 3 to 5,” Yoshimura said at a media briefing, adding, however, that “we have to pay special attention” to the possibility of worsening due to hemorrhage after the procedure.
Addressing the trade-off between risks and benefits, Tudor Jovin, MD (Cooper University Health Care, Camden, NJ), who was not involved in the study, said that’s no different than considerations around use of IV tissue plasminogen activator (tPA) or EVT in smaller cerebral infarcts. “We know that this is the price to pay,” he told TCTMD. But, he added, “what matters to patients is that there is a much higher chance of having a good outcome, and I think most patients will accept this extra risk of intracerebral hemorrhage if there is an even higher chance of having a better neurological outcome.”
RESCUE-Japan LIMIT
The trial, conducted at 45 centers in Japan, enrolled 203 patients (mean age 76 years; 56% men) with acute ischemic strokes caused by large-vessel occlusions and with large ischemic cores (ASPECTS 3 to 5). “We did not evaluate patients with an ASPECTS value of 2 or lower because they have extensive infarction and are unlikely to regain functional independence,” the authors explain.
The median NIHSS score at baseline was 22, and the median ASPECTS was 3 in the EVT arm and 4 in the control arm. Overall, 28% of patients received IV tPA. The technique used for EVT was left to the discretion of the treating physicians and could include use of stent retrievers, aspiration catheters, balloon angioplasty, intracranial stents, and carotid-artery stents.
The advantage for EVT in terms of the primary outcome—mRS score of 0 to 3 at 90 days—was seen across subgroups defined by age, time to hospitalization or randomization, baseline NIHSS score, and use of tPA.
Although there were no significant differences in the proportion of patients who achieved mRS scores of 0-2 or 0-1, an analysis of the distribution of mRS scores in the two arms indicated a significant benefit with EVT (common OR 2.42; 95% CI 1.46-4.01). In addition, patients who received the intervention were more likely to have an improvement in NIHSS score of at least 8 points at 48 hours (31.0% vs 8.8%; RR 3.51; 95% CI 1.76-7.00).
Any ICH was more frequent with EVT, but rates of other safety outcomes—including death or a recurrence of cerebral infarction at 90 days and decompressive craniectomy within 7 days—did not differ significantly between groups.
Yoshimura acknowledged some limitations of the trial, including the use of MRI for diagnosis in the vast majority of patients, which may lead to differences in how ASPECTS is calculated compared with CT; the possibility of errors in calculating ASPECTS in the emergency settings; the open-label design; and the uncertain generalizability outside of the Japanese population.
Several Trials Ongoing
Still, Jovin commented, the question of whether large infarcts should preclude EVT is a hot topic within the field. Several RCTs are ongoing, and he said that RESCUE-Japan LIMIT is the first to provide some insights.
The fact that EVT provided better outcomes in this trial is not a surprise, Jovin said, adding, however, that “what is surprising to me is the magnitude of the benefit, which is similar to what we see in the moderate or small baseline infarcts. And it really begs the question whether we should really care about the size of the baseline infarct when we take these patients for thrombectomy.”
Nonetheless, results from additional trials will important, with Jovin cautioning against interpreting this one trial with a relatively small number of patients from a single country as proof that EVT is the way to go in treating these types of patients. “I think it would be important that all other ongoing randomized trials continue so that we have, in fact, a definitive answer to this question,” Jovin said.
Yoshimura expressed optimism at the end of his presentation, saying: “We hope more patients with large infarctions will be saved by receiving EVT based on this trial.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Yoshimura S, Sakai N, Yamagami H, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2022;Epub ahead of print.
Disclosures
- RESCUE-Japan LIMIT was supported in part by the Mihara Cerebrovascular Disorder Research Promotion Fund and the Japanese Society for Neuroendovascular Therapy.
- Yoshimura reports research grants from Stryker, Siemens Healthineers, Bristol-Myers Squibb, Sanofi, Eisai, Daiichi Sankyo, Teijin Pharma, Chugai Pharmaceutical, HEALIOS, Asahi Kasei Medical, Kowa, and CSL Behring; and lecture fees from Stryker, Medtronic, Johnson & Johnson, Kaneka, Terumo, Biomedical Solutions, Boehringer-Ingelheim, Daiichi Sankyo, Bayer, and Bristol-Myers Squibb.




Comments