Etripamil Nasal Spray Gains FDA Approval for Acute Paroxysmal SVT
The self-administered treatment rapidly converts patients into sinus rhythm and may reduce trips to the hospital.

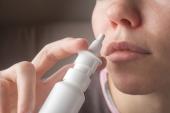
The US Food and Drug Administration has approved etripamil nasal spray (Cardamyst) for adults with acute symptomatic episodes of paroxysmal supraventricular tachycardia (PSVT), drugmaker Milestone Pharmaceuticals announced late last week.
The self-administered treatment, expected to hit pharmacy shelves in the first quarter of 2026, is an L-type calcium channel blocker designed to rapidly stop episodes of PSVT and restore sinus rhythm.
The approval was based on the results of a clinical trial program that included more than 1,800 patients and more than 2,000 episodes of PSVT. In the phase III RAPID trial, patients using the etripamil spray were more likely to convert from SVT to sinus rhythm within 30 minutes than those who used a placebo spray (64.3% vs 31.2%; HR 2.62; 95% CI 1.66-4.15). The median time to conversion was shorter in the etripamil group (17 vs 54 minutes).
Accumulated clinical data suggest that patients using the nasal spray are less likely to seek medical interventions or head to the emergency department.
Safety and efficacy with etripamil are consistent across subgroups, including those treated with beta-blockers or calcium channel blockers, according to Milestone Pharmaceuticals. Common adverse events include local-site nasal discomfort, nasal congestion, rhinorrhea, throat irritation, and epistaxis. Fewer than 2% of participants in the clinical studies discontinued due to adverse events.
The company, following the success of the ReVeRA-201 phase II trial, plans to start a phase III program to evaluate etripamil nasal spray in patients with atrial fibrillation and a rapid ventricular rate. If successful, it will follow the supplemental new drug application regulatory approval pathway to seek an added indication, with support from the PSVT approval and data from the PSVT program.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Milestone Pharmaceuticals. Milestone receives FDA approval of Cardamyst (etripamil) as first and only self-administered nasal spray for adults with paroxysmal supraventricular tachycardia (PSVT). Published on: December 12, 2025. Accessed on: December 15, 2025.




Comments