FDA Clears Next-Generation Sapien 3 Ultra TAVR Valve
This latest-iteration transcatheter heart valve features design changes and a new delivery system.
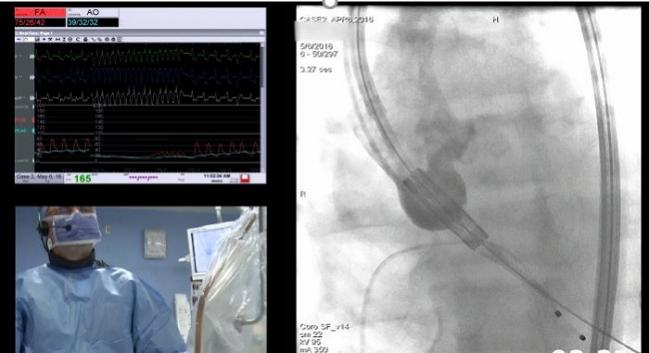
The Sapien 3 Ultra transcatheter heart valve has been cleared by the US Food and Drug Administration, device maker Edwards Lifesciences announced last week.
The valve is indicated for patients with severe, symptomatic aortic stenosis and an intermediate or higher surgical risk.
In a press release announcing CE Mark approval for the device back in November, the company said the next-generation system, which comes with valve sizes of 20, 23, and 26 mm, “features enhancements to the valve, and a new delivery system and sheath. The valve has a heightened outer skirt designed to eliminate paravalvular leak, while a revamped, 14-French delivery system can be used for all valve sizes and features an ‘on balloon’ design, “removing the need for valve alignment during the procedure,” the company notes.
In a presentation discussing the design features at the TVT 2017 meeting, David A Wood, MD (Vancouver General Hospital, Canada), suggested that the newer design would allow nine out of 10 patients to undergo “fully awake” procedures in under 30 minutes and be safely discharged the same day, or the next. Changes to the delivery system lower the crossing profile for the device so that balloon aortic valvuloplasty is less often required, Wood noted, while a “responsive articulation” means that there is less contact with the aortic wall.
Photo Credit: Adapted from: Wood DA. SAPIEN 3 Snapshots I: The Edwards SAPIEN 3 Ultra System. TVT 2017. Presented June 16, 2017. Chicago, IL.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Edwards Lifesciences. Edwards’ Sapien 3 Ultra transcatheter heart valve receives FDA approval. Published on: December 28, 2018. Accessed on: January 2, 2019.


Comments