FDA Warns Against Off-Label Use of Balloon Angioplasty to Treat Autonomic Dysfunction
With no scientific evidence, the transvascular procedure and claims of efficacy by one operator seem like ‘pseudoscience,’ a neurologist says.
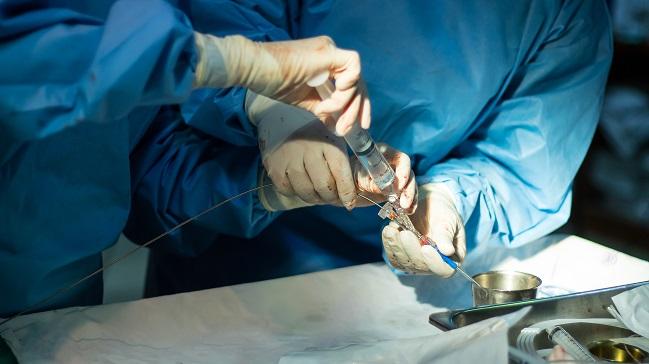
Citing a lack of outcomes research on the safety and efficacy of an experimental procedure using balloon angioplasty to treat autonomous dysfunction associated with a variety of neurologic conditions, the US Food and Drug Administration (FDA) today is warning patients and practitioners against its use.
Transvascular autonomic modulation (TVAM) is a process similar to coronary angioplasty by which an operator inserts a catheter into a patient’s venous system, primarily the jugular vein, and inflates a balloon. Akin to controversial treatments for multiple sclerosis proposed by Italian researcher Paolo Zamboni, MD (Azienda Ospedaliero-Universitaria di Ferrara), in 2006, TVAM has not been reviewed by the FDA and requires the off-label use of several devices only approved for arterial settings.
The FDA’s safety communication, published today, singles out Michael Arata, MD (Autonomic Specialists, Newport Beach, CA)—an interventional radiologist who advertises this procedure to patients primarily with multiple sclerosis but also other diseases—for endorsing the safety and efficacy of the procedure without scientific proof. Arata did not respond to TCTMD’s requests for comment, but his website asserts that “most patients have experienced substantial relief” from symptoms such as fatigue, sleep disturbances, “brain fog,” thermal intolerance, headaches, bladder or bowel dysfunction, and stomach pain.
Additionally, though not described in depth, the website explains that TVAM is “thought to work via expanding the vein which activates the venous distension reflex. This expansion of the vein leads to stimulation of the autonomic nerve fibers, which runs alongside the vein.”
The FDA warns against potential risks associated with TVAM including death, blood clots, cranial nerve damage, abdominal bleeding, and balloon rupture and subsequent migration to the lung, which would necessitate surgery.
Today’s statement marks the second time the FDA has cautioned against similar procedures for neurologic disorders. In 2012, the agency published a safety communication regarding the risks associated with procedures using the same medical devices to treat chronic cerebrospinal venous insufficiency. It also sent a warning letter to Arata specifically challenging his research methodology in this field.
‘Pseudoscience’
David Thaler, MD, PhD (Tufts Medical Center, Boston, MA), a neurologist said he who had no prior knowledge of TVAM before today, told TCTMD that it “sounds like pseudoscience, which is being promoted by the person who performs the procedure and mostly benefits from increasing the volume of the procedures he can do.
This idea is barely plausible. David Thaler
“There are all sorts of steps to developing new treatments for various problems and one of them is plausibility so if things are plausible the threshold for acceptance is a bit lower. If things are implausible than the threshold is higher,” he continued. “This idea is barely plausible.”
Any support for TVAM’s purported effectiveness would stem from the fact that there are autonomic receptors in veins, especially the jugular, Thaler explained. “I guess there could theoretically be some impact transiently as a vein is being dilated on blood pressure or heart rate or something,” he said, but “it makes absolutely no sense that that would persist beyond the time the balloon is pulled out.”
While Thaler did not totally negate the possibility that TVAM might work for some patients with autonomic dysfunction, the procedure “needs to be evaluated carefully and rigorously,” he said, adding that the endpoints would need to be outcomes that could be objectively measured, unlike subjective factors like “brain fog” that are hard to verify.
“I don’t know his company, I don’t know him, and I don't know this procedure for any of these syndromes,” Thaler observed. “That in itself is an illustration of how premature it would be to make the types of claims that seem to be being made on his website.”
What is difficult in this situation is that the patients to whom TVAM might appeal often have difficult-to-treat chronic conditions who are easily convinced to try something experimental, he concluded. “Those are just the places where we have to be wary and let science protect us.”
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Food and Drug Administration. FDA concern over experimental procedures that use balloon angioplasty devices to treat autonomic dysfunction: FDA safety communication. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm545286.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery. Published and accessed on: March 8, 2017.
Disclosures
- Thaler reports no relevant conflicts of interest.


Comments