ILUMIEN III: OCT Imaging Matches IVUS but Fails to Beat Angiography for Stent Guidance in PCI
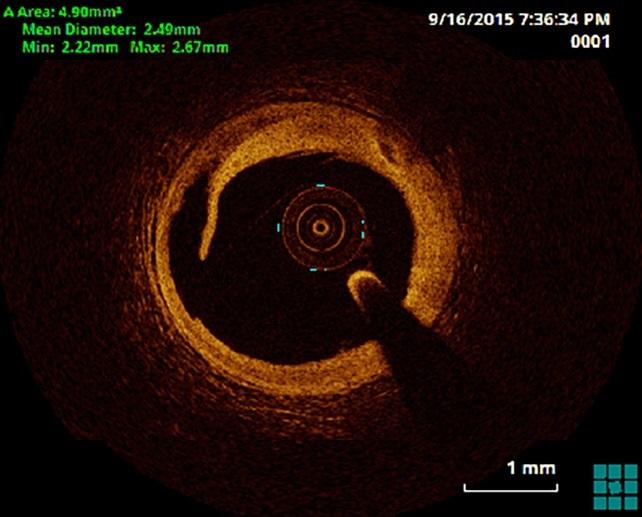
WASHINGTON, DC—A three-way comparison of optical coherence tomography (OCT), intravascular ultrasound, and angiography has come up with mixed results when it comes to choosing the best imaging modality to guide stent placement in PCI. Investigators say that while the study met its primary endpoint—OCT was noninferior to IVUS—it did not meet hierarchical tests geared to demonstrate superiority of OCT over angiography and IVUS. Experts say there are more questions that need to be answered before the many physicians who currently rely solely on angiography can be persuaded to adopt newer, high-resolution intravascular imaging.
Results of the ILUMIEN III: OPTIMIZE PCI trial, with lead author Ziad Ali, MD (NewYork-Presbyterian Hospital/Columbia University, New York, NY), were released here at TCT 2016 today and published simultaneously in the Lancet.
“For the first time we’ve shown that the minimum stent area by OCT guidance is noninferior to IVUS guidance, whereas previous studies have shown that OCT may have led to the choice of a smaller stent diameter and thus a smaller lumen,” Ali told TCTMD. “The superior resolution of OCT, which has never really been shown to provide any clear-cut benefit, we show for the first time is able to detect malapposition and major dissection that were missed by IVUS and thus could potentially impact clinical outcomes.”
Whether that proves to be the case is a question for ILUMIEN IV, he added. “ILUMIEN III was part of a series of trials. [It] was never intended to provide clinical outcomes. It would be grossly underpowered. This was designed using a novel stent sizing approach to insure that we're getting a large enough lumen area to potentially provide the benefit that has already been proven with IVUS-guided PCI.”
Imaging, Three Ways
Angiography has been the longtime workhorse for stent placement during angioplasty, but it provides only two-dimensional representation of the coronary anatomy and is limited to luminal dimensions and characteristics, without additive information on plaque morphology, vascular remodeling, burden of atherosclerosis, or nuances of stent positioning. IVUS allows cross-sectional tomographic imaging of the vessel wall that may help optimize stent placement and, in particular, lead to larger minimum stent area, which has been associated with reduced MACE in a number of studies. IVUS is not, however, routinely used by most cath labs. The latest player in this space, OCT, delivers rapid, high-resolution images that more accurately depict vessel and lesion characteristics including thrombus, calcium, fibrous cap thickness, and dissections, as well as stent malapposition and strut coverage. No previous study has attempted to compare all three modalities head-to-head.
For ILUMIEN III, Ali and colleagues randomized 450 patients undergoing PCI at one of 29 hospitals to OCT, IVUS, or angiographic guidance. Roughly one-third of patients in each arm had non-STEMI or recent STEMI, and very complex cases were excluded (left main, chronic total occlusions, in-stent restenosis, and bypass graft stenosis, among others). For the OCT group, operators used a specific protocol for establishing stent length, diameter, and expansion according to reference segment external elastic lamina measurements—an important step that the investigators believe will help minimize stent undersizing.
For the primary endpoint of final median minimum stent area (measured by OCT at a masked, independent core lab), OCT guidance was noninferior to IVUS but not superior. Nor was it superior to angiography-guided stent placement. Of note, Ali told TCTMD IVUS was superior to angiography in ILUMIEN III ”but the trial was not powered for this analysis.” Procedural MACE occurred in four patients in the OCT group, in one in the IVUS group, and in one in the angiography group, but rates were not statistically different between OCT and IVUS or between OCT and angiography.
Minimum and mean stent expansion were significantly greater with OCT-guided PCI than with angiography-guided PCI, but they were similar to IVUS-guided PCI. Procedural success was also higher with OCT than with angiography. Patients in the IVUS and angiography groups had more untreated edge dissections than did patients in the OCT group. In what may reflect the higher resolution of OCT, untreated major dissections were more common after IVUS-guided PCI than after OCT-guided PCI, and untreated major stent malapposition was more common after IVUS and angiography than it was in OCT-guided procedures.
Speaking with TCTMD, study co-author Giulio Guagliumi, MD (Ospedale Papa Giovanni XXIII, Bergamo, Italy), pointed out that when it comes to optimized stent area, IVUS is “the gold standard” so the intention in the study was to meet noninferiority, not to show superiority. “Then, collaterally, we wanted to try and see if we could be superior to angiography, and here we saw only a trend and [it was] not clearly demonstrated,” he said.
He believes that the “global representation” of patients across the spectrum of coronary artery disease severity likely diluted the ability of OCT to show a benefit over angiography. “There was no selection of patients in terms of having a high level of complexity . . . including over 60% of patients with stable lesions and silent ischemia.” He continued: “I think OCT can be of most benefit when we identify the true patient and true lesion complexity that can benefit from this technology.”
All the same, when asked whether the hope had been to demonstrate superiority to angiography in ILUMIEN III, both Guagliumi and Ali answered with different versions of: “Yes, but . . . ”
“In fact, you’re right,” Guagliumi told TCTMD. “But this is a foundation study, not a pivotal study. It was designed to show that one specific methodology that was not conventionally used can be used clinically.”
Ali, on the same question, responded, “Yes, however, OCT was superior for minimum and mean stent expansion and acute procedural success, so the minimal stent area is not the only outcome parameter associated with successful PCI. It was just the one that we powered the primary endpoint towards.
“If you look just at the primary endpoint,” he continued, “then it didn't beat angiography, although there is a trend towards superiority. But if you look at all the parameters taken together it provides better stent expansion, both mean and minimum, it provides better identification of postprocedural complications, which include major dissections, major malapposition, and tissue protrusion. So taken together, that's a win for OCT. Moreover, it provides us the basis for being able to perform a randomized clinical outcomes trial which is really what we're trying to get to.”
Impetus to Switch to Intravascular Imaging?
According to Ali, approximately 20% of US cath labs use intravascular ultrasound (it is closer to 40% at his institution) and just 8% use OCT. The number is similarly low in Europe but approaches 90% in Japan, where it is reimbursed.
Many operators, however, believe they are already getting excellent results with angiography. Ali is unconvinced.
“For those people who say, ‘My results are excellent with angiography alone,’ we have to take intravascular imaging as a whole and say, there are large randomized controlled trials and meta-analyses of up to 30,000 patients that show a clear benefit in terms of stent thrombosis, death, and target lesion revascularization that favors intravascular imaging,” he said. Data from trials like TWENTE suggest that even with the newest drug-eluting stents, target lesion revascularization is approaching 18% at 5 years. By contrast, results from intravascular imaging studies in Korea suggest rates have fallen from roughly 6% to 3% when the newer imaging modalities were employed more universally.
Still, that kind of benefit needs to be demonstrated in large, randomized, adequately powered clinical trials, as Ravinay Bhindi, MD, and Usaid K Allahwala, MD (Royal North Shore Hospital, Sydney, Australia), write in an editorial accompanying the Lancet article.
“Although we applaud and welcome the findings of the ILUMIEN III: OPTIMIZE PCI trial as an important step in the right direction, the interventional cardiology community have previously fallen into the trap of using surrogate measures to argue for the benefit of a strategy or approach which, when tested systematically, has shown no difference or change in clinical outcomes,” they say. “Although this study strengthens the evidence for intracoronary OCT use, whether or not it should be used routinely in all cases remains unclear.”
During a morning press conference, experts speaking to the press debated the increased cost of OCT over angiography, with Ajay Kirtane (NewYork-Presbyterian Hospital/Columbia University Medical Center, New York, NY), ballparking the number as approximately a $700 increased cost for the newer imaging tool, while Paul Teirstein, MD (Scripps Clinic, La Jolla, CA), called the cost difference, “not huge, not a deal-breaker.”
Ali pointed out that better characterization of the vessel wall and optimization of the stent placement may potentially translate into lower costs via less use of pre- or postdilation. Whether there are also longer-term savings related to less repeat hospitalizations or revascularizations remains to be seen.
Dean Kereiakes, MD (Christ Hospital Heart and Vascular Center Medical, Cincinnati, OH) however, noted that “on balance [intravascular imaging] drives increased utilization of resources, on average, not less.”
Note: Several co-authors are faculty members of the Cardiovascular Research Foundation, the publisher of TCTMD. Photo credit: Dr. Ali.
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Ali ZA, Maehara A, Généreux P, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomized controlled trial. Lancet. 2016;Epub ahead of print.
Bhindi R, Allahwala UK. Optical coherence tomography: Not quite ready. Lancet. 2016;Epub ahead of print.
Disclosures
- Ali reports grants and personal fees from St. Jude Medical, Acist Medical, and Cardiovascular Systems, outside the current study.
- Guagliumi reports being on the advisory board through the hospital for St. Jude Medical during the conduct of the study and reports personal fees from St. Jude Medical and Boston Scientific as well as and grants from Abbott Vascular and Boston Scientific outside the submitted work.
- Bhindi and Allahwala report no relevant conflicts of interest.


Comments