Injectable P2Y12 Inhibitor Selatogrel Shows Promise in Acute MI
With a phase III trial planned, clinical challenges over how to administer the drug to patients will need to be addressed.
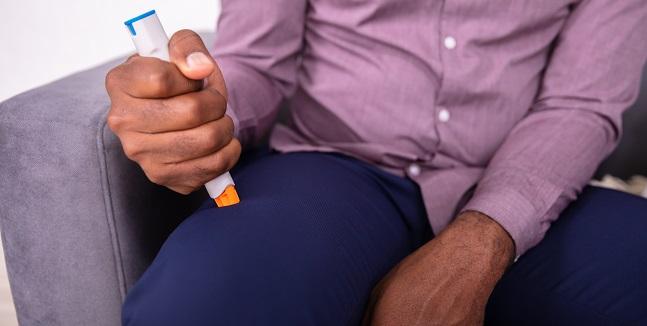
The novel reversible P2Y12 inhibitor selatogrel (Idorsia Pharmaceuticals), administered via subcutaneous injection, is safe and causes a rapid dose-related response when administered in patients with acute MI, according to results from a phase II trial.
Time is of the essence for patients with acute MI, but the primary pharmaceutical options to reduce platelet reactivity are oral, including the irreversible thienopyridines clopidogrel and prasugrel and the reversible ticagrelor, all of which need time to take effect. Intravenous cangrelor (Kengreal, Chiesi) is faster acting, and reversible, but requires continuous infusion and is typically only used during PCI in patients who have not taken—or can’t take—oral P2Y12 inhibitors or as a bridge to surgery.
A subcutaneous, injectable option, in this case often followed by a ticagrelor boost, could potentially shorten the period between symptom onset and platelet inhibition, which has shown to improve coronary reperfusion before PCI and reduce the risk of recurrent events over time.
“Selatogrel will be developed as drug to be self-administered subcutaneously by the patient,” senior study author Marco Valgimigli, MD, PhD (Cardiocentro Ticino, Lugano and University of Bern, Switzerland), told TCTMD in an email. “No drug or treatment option is currently available in this space so, if granted, selatogrel will have a unique indication.”
Of course, this kind of patient-administration model will face challenges—namely, educating patients as to how and when to inject and informing providers which patients are the best candidates for selatogrel prescriptions—but Valgimigli said further research should help in this regard. “In particular, education should be interpreted in both directions,” he said. For healthcare providers “the first step would be to inform the patient on this possible new treatment option [and] to be able to target patients who understand the value and possible risks of the treatment and be able to obtain full commitment from them. The involvement of [general practitioners] or referral cardiologists will be probably key.”
The drug is a highly selective and potent 2-phenylpyrimidine-4-carboxamide analog that can reversibly bind with P2Y12 receptor antagonists. Prior research in healthy adults showed a quick and robust antiplatelet effect. Given that it directly inhibits P2Y12, Valgimigli said “it should not suffer from resistance or poor responsiveness” as has been observed with clopidogrel.
The paper was published in the May 26, 2020, issue of the Journal of the American College of Cardiology.
16-mg Dose Offers Consistent Effect
For the study, Peter Sinnaeve, MD, PhD (University Hospitals Leuven, Belgium), Valgimigli, and colleagues enrolled 47 patients (median age 69 years) with acute MI (62% STEMI) from six sites in Belgium, Switzerland, and Israel between July and November 2018. Participants were randomized to receive either an 8-mg (n = 24) or 16-mg (n = 23) dose of selatogrel followed by ticagrelor (n = 43) or clopidogrel (n = 1).
Using VerifyNow measurements available in 45 patients at 30 minutes following selatogrel injection, 91% and 96% of patients who received the 8-mg (P = 0.142) and 16-mg doses (P = 0.009), respectively, responded to the drug (primary endpoint; defined as PRU < 100). These response rates did not vary by presentation, age, or sex. Similar responses were noted at 15 minutes (75% and 91%), 30 minutes (88% and 91%), and 60 minutes (75% and 96%) in the full cohort of 47 patients (P = 0.006).
Half of the 8-mg group and 35% of the 16-mg group reported at least one treatment emergent adverse event, with ventricular tachycardia being the most common but only serious in two patients. Mild dyspnea deemed related to the injection was also reported in one patient who received the 16-mg dose.
Given these results, Valgimigli said that the 16-mg dose of selatogrel will be used in phase III trials. “The plan is to develop selatogrel as a drug to be self-administered by patients who suspect to be suffering from an ongoing cardiac attack,” he said. “Patients who already had a prior heart attack and remain at high risk for recurrences will be targeted. If the use of the drug could be offered also to patient subsets at lower risk of heart attack will have to be seen pending the results of the pivotal approval study, which will be called SOS AMI.”
Clinical Challenges
In an accompanying editorial, Johanne Silvain, MD, PhD (Hôpital Pitié-Salpêtrière, Paris, France), and colleagues write that the use of a loading dose of ticagrelor in most patients “makes it difficult to apprehend the pharmacodynamic effect attributed to selatogrel itself. . . . However, a strong P2Y12-mediated platelet inhibition could be noted as fast as 15 minutes after the injection of the 16-mg dose of selatogrel, with data on pharmacokinetic showing that selatogrel plasma concentration peaked at 30 minutes for the 16-mg dose and at 60 minutes for the 8-mg dose.”
They say the upcoming phase III trial is “exciting” but brings up many a clinical challenge, including: “How does one educate the right patient to be able to administer the drug at the right time to avoid differential diagnosis? What is the risk of bleeding associated with self-administration of selatogrel in patients already treated by an antiplatelet drug without confirmed diagnosis of acute MI? Will the pharmacological specificity of selatogrel—fast action and short effect in time—be sufficient to provide a net clinical benefit in all coronary patients suspected with a recurrent acute MI?”
Prior research has shown the benefit of fast and strong platelet inhibition among STEMI patients, it remains unclear in a population of patients with NSTEMI, the editorialists add. “For now, we just know that this novel class of P2Y12 inhibitor has the potential to save time in terms of early platelet inhibition.”
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Sinnaeve P, Fahrni G, Schelfaut D, et al. Subcutaneous selatogrel inhibits platelet aggregation in patients with acute myocardial infarction. J Am Coll Cardiol. 2020;75:2588-2597.
Silvain J, Zeitouni M, Kernis M. Selatogrel for acute myocardial infarction: the promise and challenges of self-medication. J Am Coll Cardiol. 2020;75:2598-2601.
Disclosures
- This study was sponsored by Idorsia Pharmaceuticals.
- Sinnaeve reports receiving consultancy and/or speaker fees and grants from AstraZeneca and Daiichi-Sankyo all of which were collected institutionally; and serving as a clinical investigator for the Fonds voor Wetenschappelijk Onderzoek—Vlaanderen.
- Silvain reports receiving consulting fees, lecture fees, or travel support from AstraZeneca, Bayer HealthCare SAS, Boehringer Ingelheim France, CSL Behring SA, Gilead Science, Sanofi France, Terumo France SAS, Abbott Medical France SAS, Biotronik, and Zoll; and is a stockholder of Pharmaseeds.


Comments