LRT Trial Data Bolster Support for TAVI in Bicuspid Valves
Outcomes and hemodynamics at 1 year were reassuring, but long-term data in these often-younger patients will be important.
TAVI in low-risk bicuspid aortic stenosis can result in good outcomes out to 1 year, with valve performance comparable to that seen in tricuspid patients and a low risk of death, according to data from a subset of bicuspid patients included in a multicenter trial.
“Reassuringly clinical outcomes and TAVR valve hemodynamics remained excellent for 1 year,” said Toby Rogers, MD, PhD (MedStar Heart & Vascular Institute, Washington, DC), in a late-breaking trial session at the virtual CRT 2021 meeting. The rate of all-cause mortality at 1 year was 1.5% in the bicuspid group versus 3.0% in similar patients with tricuspid valves who underwent TAVI, he reported during his presentation.
The data come from the Low-Risk TAVR (LRT) trial, which included a separate substudy of 72 patients with bicuspid aortic stenosis treated with transfemoral TAVI who were enrolled between 2016 and 2020. For the analysis, the bicuspid TAVI patients were compared with a matched bicuspid cohort from the same institutions who underwent surgery and the 197 low-risk patients with tricuspid aortic stenosis treated with TAVI in the main LRT trial.
“I think it's encouraging and adds to the evidence that we're generating in treating patients with truly bicuspid aortic valves with the TAVR procedure,” said Colin Barker, MD (Vanderbilt University Medical Center, Nashville, TN), who commented on the findings for TCTMD.
Higher Rates of Nondisabling Stroke and New Pacemaker
Although bicuspid TAVI patients were younger than tricuspid TAVI patients (mean age 68 vs 74 years), bicuspid SAVR patients were even younger, with a mean age of 63 years. Regardless of the type of intervention, bicuspid patients had fewer comorbidities across the board than tricuspid patients.
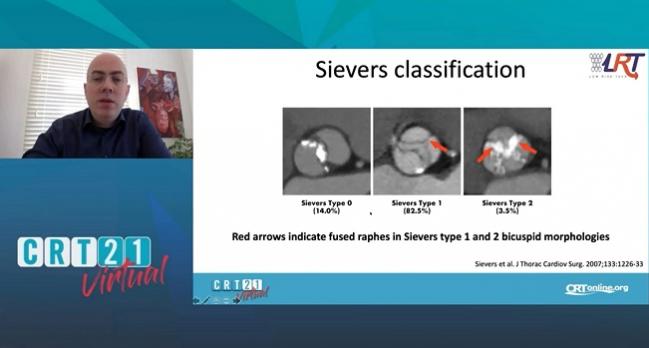
Sievers classification, which was used to categorize bicuspid anatomy and morphologies, showed that 82.5% were Type 1 and fewer than 15% were Type 0. Rogers said those findings align with other bicuspid patient data including those from the Evolut Low Risk study and PARTNER 3. Bicuspid patients requiring replacement of the ascending aorta were not included in the TAVI group of the LRT trial.
Valve choice was at the operator’s discretion, with two-thirds of bicuspid patients receiving a balloon-expandable valve and the others a self-expanding valve. Nearly 40% of bicuspid TAVI valves were 26 mm, and 33.3% were 29 mm. In comparison, most bicuspid SAVR valves were 23 mm (43%).
In the bicuspid cohort, one patient died (out of the 67 who had complete 1-year follow-up). By comparison, six patients out of the 197 followed in the tricuspid TAVI group died within 1 year.
“Like many studies, COVID definitely interfered with our ability to get good, complete follow-up datasets,” Rogers acknowledged in his presentation. Data on bicuspid SAVR was not available beyond 30 days.
The rate of stroke at 1 year in the bicuspid TAVI group was 4.5%, all of which were adjudicated as nondisabling. This compared with a stroke rate of 2.1% in the tricuspid TAVI group. New permanent pacemakers were required in 13.6% of bicuspid TAVI patients and in 7.3% of the tricuspid TAVI group. At baseline, just under 5% of the bicuspid cohort was in NYHA class I. By 1 year, 84.1% were in class I, up from 78.3% at 30 days.
In terms of hemodynamics, the mean aortic valve gradient at baseline was 55.0 mm Hg, decreasing to 15.1 mm Hg at discharge and 13.2 mm Hg by 1 year. Mean aortic valve area, which was 0.7 cm2 at baseline, rose to 1.9 cm2 at discharge and was 1.7 cm2 by 1 year.
Of 61 patients who underwent 4-D contrast-enhanced CT at 30 days to assess for subclinical leaflet thrombosis, 10.2% had hypoattenuated leaflet thickening (HALT), 6.9% had reduced leaflet motion (RELM), and 6.9% had hypoattenuation affecting motion (HAM).
Longer Follow-up Needed
Barker said while the findings are “very reassuring,” they don’t tell the whole story about the differences between bicuspid and tricuspid patients. He added that much more needs to be teased out.
“Interestingly, there were more women in this group than men, which is not necessarily consistent with what we see with bicuspid pathology in general,” he noted. “The pacemaker rate is also a little higher than we would expect to see in a younger population.” Since true bicuspid patients tend to have more heavily calcified valves than patients with tricuspid valves, Barker said that could account for some of the higher pacemaker need.
The other issue is the higher risk of nondisabling stroke. While not alarming, it is something that physicians and patients need to consider carefully and will only be fully understood with long-term follow-up, Barker noted.
“Any amount of stroke in a younger person could be harmful long-term,” he said. “Embolic protection may or may not sort its way out and prove to be beneficial in specific populations like bicuspid, where there is in general a little bit of a higher stroke risk. Whether it's disabling or not, it is a complication, and it’s something we as physicians want to avoid and patients especially want to avoid.”
As for the 10% of patients with evidence of HALT after the procedure, Barker said that is another thing that needs to be followed long-term, calling the situation “somewhat nebulous.”
“We sort of empirically treat it. Some of us do, some don't. We definitely do if there's a hemodynamic or physiologic effect,” he said, adding that he typically manages those patients on an anticoagulant and reevaluates in 3 months with repeat imaging.
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Rogers T. Transcatheter Aortic Valve Replacement in Low Risk Patients with Bicuspid Aortic Stenosis: 1-year results from the LRT trial. Presented at: CRT 2021. March 6, 2021.
Disclosures
- Rogers reports consulting and serving as a physician proctor for Edwards Lifesciences and Medtronic; serving on the advisory board of Medtronic; and having equity interest in Transmural Systems.
- Barker reports consulting for Edwards Lifesciences.





Comments