LuX-Valve TTVR Device Provides Favorable 1-Year Results
Where this device will fit in with other emerging options remains to be seen, but it might hold an advantage in large annuli.
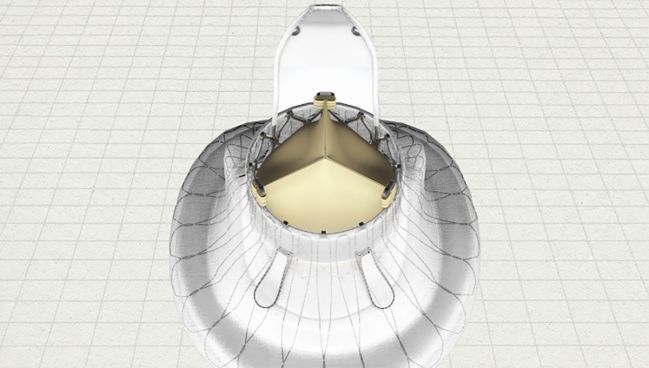
Photo Credit: Jenscare Biotechnology
One-year follow-up data suggest that a novel transcatheter tricuspid valve replacement (TTVR) device may provide another potential alternative to surgery for patients with severe tricuspid regurgitation (TR).
In the TRAVEL study, published this month in JACC: Cardiovascular Interventions, Xiangbin Pan, MD, PhD (Fuwai Hospital, Beijing, China), and colleagues show a sustained reduction in TR among patients treated with the LuX-Valve (Jenscare Biotechnology), with 95.2% reporting mild or less regurgitation at 1 year.
The device is composed of a nitinol frame with a self-expanding atrial disc, three bovine pericardium leaflets, two graspers, and a tongue-shaped anchor. The first-generation bioprosthesis used in the new study is delivered transatrially with guidance from transesophageal echocardiography (TEE) and intraoperative fluoroscopy, but the manufacturer has since announced its LuX-Valve Plus system, which can be implanted transjugularly.
“The LuX-Valve may offer an acceptable treatment option for high-risk patients with TR who have limited alternative therapeutic options,” the authors write.
Indeed, the once-empty toolbox is getting more crowded for these patients who aren’t eligible for surgery. Last year, the US Food and Drug Administration approved the Evoque tricuspid valve replacement system (Edwards Lifesciences) as the first TTVR device for this patient cohort, and last month the US Centers for Medicare & Medicaid Services (CMS) agreed to reimburse for the procedure. The field of tricuspid transcatheter edge-to-edge repair (T-TEER) is also evolving, with CMS currently considering coverage options.
In an accompanying editorial, Neil P. Fam, MD, and Sami Alnasser, MD (both St. Michael’s Hospital, University of Toronto, Canada), write that this “expansion of the TTVR size matrix and design diversity should be very exciting to the field. Because tricuspid valve anatomy and related TR mechanisms are highly variable, ongoing innovation is essential to enable the treatment of more patients in need of tricuspid valve intervention.”
Steven Yakubov, MD (Riverside Methodist Hospital, OhioHealth, Columbus), who was not involved in the study, agreed. He told TCTMD that he foresees about a dozen orthotopic devices used to treat TR percutaneously coming down the pipeline, with maybe three or four eventually making it to market. For now, the results of the TRAVEL study are “ interesting, promising, and certainly a reason to keep moving forward,” he said.
Sustained TR Reductions
The single-arm study included 126 patients (mean age 65.8 years; 79.4% women) who were deemed to be high risk and were treated between June 2020 and August 2021. All patients had NYHA class III or IV symptoms at baseline.
The LuX-Valve was delivered transatrially in all cases. Procedural success was achieved in 97.6% of patients, with one requiring conversion to sternotomy due to RV perforation. Just over one in 10 patients reported procedure-related access-site bleeding, and 1.6% of patients required a new pacemaker.
At 1 year, 10.3% of patients had died and 4.0% had been hospitalized for heart failure. Along with the reductions in TR (75.7% reported none/trace TR), the researchers observed 38.3- and 6.4-mL drops in right atrial systolic volume and mid right ventricular end-systolic volume, respectively. Most patients (79.8%) had symptoms classified as NYHA class I or II and mean 6-minute walking distance increased by 71.3 m (P < 0.001 for all).
Pan and colleagues outline some hurdles ahead for TTVR, including the identification of proper candidates for the procedure, careful selection of devices to prevent unnecessary oversizing and potential associated consequences, and reductions in the need for new pacemakers and interactions with existing ones.
The LuX-Valve holds promise, they continue, because of its “conformable” atrial disc and repositionability that might prevent paravalvular leak, as well as its graspers that can indicate anterior leaflet capture. The authors note that the second-generation device, which is currently being studied in the TRINITY study, will require a less traumatic procedure since it can be deployed through the transjugular vein. “This also ensures the wider clinical application of this technology,” Pan et al write.
Future Challenges
Fam and Alnasser highlight that, due to the study’s single-arm design, the performance of the valve compared to medical therapy remains unknown. They also call for longer-term data to judge durability.
It’d be nice if somebody could actually prove that there is a mortality benefit to tricuspid replacement. Steven Yakubov
As for where the LuX-Valve might fit in with the emerging glut of options to treat TR, Yakubov said it will be best suited for patients with large annuli due to its septal anchoring mechanism. But the TTVR devices that will eventually succeed will be the ones that can be deployed with less imaging and, ultimately, under conscious sedation, he said, adding that low pacemaker interaction would be desirable, too.
With the TEE used in TRAVEL, the LuX-Valve doesn’t yet fit in that category, Yakubov said. “If a device is simple to use, [doesn’t] require general anesthesia and transesophageal echo, [and] could even be done just with intracardiac echo or surface echo in angiography, that would be a great advance.”
Also, access could be a challenge for this device and others. “Most interventionists would prefer to come from the transfemoral approach rather than being at the head of the bed with internal jugular access,” Yakubov said. “Internal jugular access is accepted by many sites, but it won’t be by all.”
The TRAVEL study represents a step in the “right direction,” he continued. “The next thing will be complete elimination of TR as well as an improvement in mortality. It’d be nice if somebody could actually prove that there is a mortality benefit to tricuspid replacement.”
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Pan X, Lu F, Wang Y, et al. Transcatheter tricuspid valve replacement with the novel system: 1-year outcomes from the TRAVEL study. J Am Coll Cardiol Intv. 2025;Epub ahead of print.
Fam NP, Alnasser S. The evolving landscape of TTVR: embracing the LuXury of choice. J Am Coll Cardiol Intv. 2025;Epub ahead of print.
Disclosures
- The study was supported by the Development and Transformation of New Technology and Construction of Precision Diagnosis and Treatment System for Transcatheter Interventional Diagnosis and Treatment of Structural Heart Diseases, the National Key R&D Program of China, and Research on Key Techniques of Minimally Invasive Treatment for Valvular Heart Diseases.
- Pan, Alnasser, and Yakubov report no relevant conflicts of interest.
- Fam reports serving as a consultant to Edwards Lifesciences, Abbott, Medtronic, and Venus Medtech.




Comments