Modest Gains With Omecamtiv Mecarbil in Chronic HFrEF: GALACTIC-HF
With several established HF therapies available, the question is where a cardiac myosin activator might fit and how much it adds.
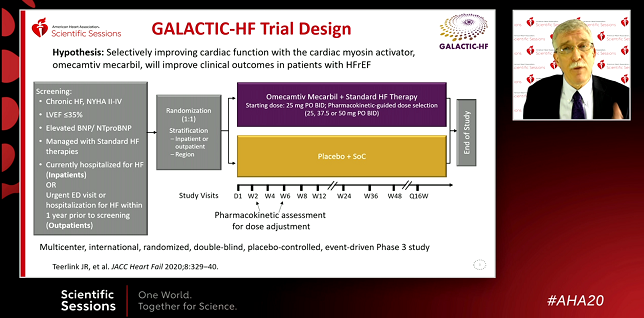
When added to standard therapies, omecamtiv mecarbil—a novel cardiac myosin activator, or myotrope—significantly reduces the combined risk of first heart failure events or CV death in patients with chronic heart failure and reduced ejection fraction (HFrEF), full results of the placebo-controlled GALACTIC-HF trial show.
The benefit was modest, with an absolute reduction of 2.1% and a relative reduction of 8% over a median follow-up of about 22 months. Also, there was no significant benefit of omecamtiv mecarbil on either of the components of the primary composite endpoint when considered individually.
Nevertheless, “for the first time, these results confirm the hypothesis that selectively increasing cardiac function with a cardiac myosin activator such as omecamtiv mecarbil can improve clinical outcomes in patients with heart failure and reduced ejection fraction,” John Teerlink, MD (University of California, San Francisco), chair of the trial’s executive committee, reported during the virtual American Heart Association (AHA) 2020 Scientific Sessions.
The findings were published simultaneously online in the New England Journal of Medicine. Amgen, Cytokinetics, and Servier, the companies involved in developing omecamtiv mecarbil, had released top-line results last month.
A Long Journey
Teerlink described the trial as the end of a very long journey. “The central defect and initiating factor in heart failure with reduced ejection fraction, or HFrEF, is a decrease in systolic function,” he said during a press briefing. “Yet despite extensive investigative efforts for over a century, no therapies for chronic heart failure with reduced ejection fraction that targeted this systolic dysfunction have improved patient outcomes, and in fact most have actually increased mortality.”
Omecamtiv mecarbil represents a new approach in that it “augments cardiac contractility by selectively binding to cardiac myosin, thus increasing the number of force generators (myosin heads) that can bind to the actin filament and initiate a power stroke at the start of systole,” the investigators explain in their paper. The drug improved cardiac function in patients with chronic HFrEF in the phase II COSMIC-HF study.
The phase III GALACTIC-HF trial, conducted at centers in 35 countries, was designed to assess omecamtiv mecarbil’s effects on clinical outcomes. The study involved 8,256 patients (mean age 66; 21% women) with chronic NYHA class II to IV disease, an LVEF of 35% or lower, and elevated natriuretic peptides. All patients were receiving standard heart failure therapies and were either currently hospitalized for HF or had experienced an urgent emergency department visit or hospitalization for HF in the year prior to screening.
Patients randomized to omecamtiv mecarbil were started at a dose of 25 mg twice daily, with some patients receiving pharmacokinetic-guided maintenance doses of 37.5 and 50 mg twice daily.
The primary composite outcome of first HF events (hospitalization or urgent visits for HF) or CV death occurred in 37.0% of patients treated with omecamtiv mecarbil and 39.1% of those who received placebo (HR 0.92; 95% CI 0.86-0.99). There was no significant benefit seen for either first HF events (HR 0.93; 95% CI 0.86-1.00) or CV death (HR 1.01; 95% CI 0.92-1.11) individually.
Omecamtiv mecarbil did not have a significant impact on the change in the Kansas City Cardiomyopathy Questionnaire total symptom score or on blood pressure, renal function, or potassium homeostasis. Compared with placebo, the drug led to greater reductions in heart rate and NT-proBNP level. Treatment also increased median cardiac troponin I level by 4 ng/L, a magnitude similar to what is seen with rigorous exercise, Teerlink said.
There were no differences in adverse events between trial arms.
Where Will It Fit In?
After Teerlink’s presentation during the press briefing, the conversation turned to where omecamtiv mecarbil will fit into treatment plans considering that there are already several other therapies with proven survival benefits in HFrEF identified in the 2017 US heart failure guidelines, including ACE inhibitors/ARBs/angiotensin receptor-neprilysin inhibitors, beta-blockers, mineralocorticoid receptor antagonists, and implantable devices.
Plus, as discussant Paul Heidenreich, MD (Stanford University School of Medicine and VA Palo Alto Health Care System, CA), noted, recent years have seen positive trial results from the sodium glucose co-transporter 2 (SGLT2) inhibitors in patients with heart failure. “It remains to be investigated and discussed about exactly where omecamtiv mecarbil will fit in,” he said.
But, Heidenreich said, “it likely has a role in multiple settings, particularly given its lack of reduction in blood pressure. Many of our other therapies do reduce blood pressure. Many of our patients are unable to take many of these medications and would benefit from additional therapy, and we just can’t provide it because of side effects.”
Importantly, he said, there were no major safety issues identified, and it’ll be interesting to explore whether there are subgroups of patients in which the benefits of omecamtiv mecarbil are greater than seen in the overall trial.
Teerlink noted during his presentation that there appeared to be a greater benefit in terms of the primary outcome in patients with an LVEF of 28% or lower (HR 0.84; 95% CI 0.77-0.92). “Given omecamtiv mecarbil’s mechanism of action of increasing cardiac function, there is biological plausibility that there may be greater benefit in patients with more substantially reduced systolic function and [that suggests] that there may be patient populations who might derive even greater benefit from this therapy,” he said.
The real advantage of the drug “is that it doesn’t compete with any of the other current therapies,” Teerlink said. “In terms of difficulty of use, it should be easy from that standpoint.”
Regardless of how all of the available options will be used together, former AHA President Clyde Yancy, MD (Northwestern University Feinberg School of Medicine, Chicago, IL), who chaired the press briefing, expressed optimism about the future of HF treatments. “I wish I could give everyone a crisp answer and say we’ve already figured out algorithmically where this is going to go, but I won’t try to do that,” he said. “I will celebrate the fact that we’ve got many more choices than we’ve ever had before. And as I’ve said in the past, this is yet another new day in heart failure.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Teerlink JR, Diaz R, Felker GM, et al. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2020;Epub ahead of print.
Disclosures
- GALACTIC-HF was funded by Amgen, Cytokinetics, and Servier.
- Teerlink reports research grants/consulting fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cytokinetics, Medtronic, Merck, Novartis, and Servier.
- Heidenreich reports no relevant conflicts of interest.


Comments