More INR Test Strips Recalled Due to Inaccurately High Results
The action follows an earlier recall of more than 1.1 million packages of CoaguChek XS PT test strips.
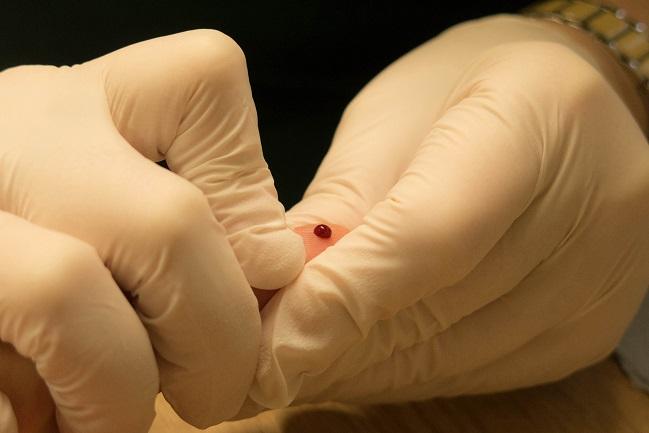
A new recall has been issued CoaguChek XS PT test strips, used to monitor international normalized ratio (INR) in patients taking warfarin, due to inaccurately high readings.
Roche Diagnostics initiated a recall of more than 1.1 million packages of the test strips in September, an action the US Food and Drug Administration categorized as a class I recall, the most serious type, in November.
Now, in a related action announced Thursday on the FDA website, all lots of CoaguChek test strips distributed by Terrific Care/Medex Supply are being recalled.
Roche Diagnostics started receiving reports of abnormal INR readings using the test strips after it recalibrated the strips to a new international standard. A falsely high INR result could lead to a reduction in warfarin dose, interruption of treatment, or administration of vitamin K.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Terrific Care, LLC/Medex Supply Dist, Inc. Terrific Care, LLC/Medex Supply Dist, Inc. issues nationwide recall of CoaguChek test strips. Published on: December 20, 2018. Accessed on: December 21, 2018.


Comments