Occluding the LAA During Surgery Lowers Stroke Risk: LAAOS III
Calling the trial conclusive, a researcher says, “We have a safe, effective intervention that can be done at low cost, low risk for patients.”
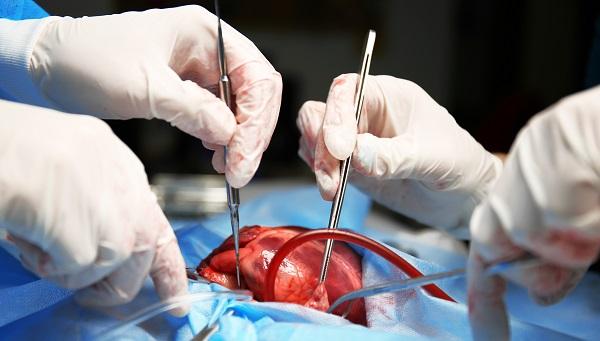
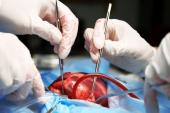
(UPDATED) Patients with atrial fibrillation (AF) who are undergoing cardiac surgery for another indication should have their left atrial appendage (LAA) occluded while they’re on the table, the LAAOS III trial indicates.
On the background of relatively high use of oral anticoagulation, such a strategy resulted in an ischemic stroke/systemic embolism rate of 4.8%, significantly lower than the 7.0% rate seen in patients whose LAA was left alone (HR 0.67; 95% CI 0.53-0.85), Richard Whitlock, MD, PhD (McMaster University, Hamilton, Canada), reported Saturday as a late-breaking clinical trial during the virtual American College of Cardiology 2021 Scientific Session.
The results, published simultaneously online in the New England Journal of Medicine, offer the first definitive proof that surgical occlusion of the LAA lowers ischemic stroke risk in patients with AF, according to Whitlock. He told TCTMD he was surprised by the magnitude of the benefit, which was even greater if events occurring in the perioperative period were excluded.
“Based on LAAOS III, if you have atrial fibrillation and an elevated stroke risk and you’re having heart surgery for some other reason, your left atrial appendage should be taken off,” he said. “And I think that’s conclusive. I think this will change guidelines.” Of note, current US and European guidelines provide a class IIb recommendation indicating that surgical LAA occlusion at the time of cardiac surgery may be considered in patients with AF.
Mathew Williams, MD (NYU Langone Health, New York, NY), also was surprised by the effect size in the study. “It’s impressive, and it’s exciting to finally see some strong data behind this because it’s something that a lot of people do, but not routinely,” he commented to TCTMD. “To now actually see some data that there’s benefit early but in particular that there’s some late effects [in terms of] reducing these embolic events is impressive.”
As for the potential impact on practice and how surgical LAA occlusion is deployed, Williams said, “I think there’s a compelling argument now that it should become more standard.”
Discussing the results after Whitlock’s presentation, Kalyanam Shivkumar, MD, PhD (UCLA Health, Los Angeles, CA), said that LAAOS III, which answered an important clinical question, “is for sure going to influence our guidelines.” He highlighted the fact that surgical LAA occlusion reduced stroke risk when added to relatively high use of oral anticoagulation, noting, “It is pretty remarkable in this group of patients that you see additive benefit for a nonpharmacologic intervention.”
A Definitive Test
Although surgical LAA occlusion during cardiac surgery in patients with AF has been used by some based on the belief that it lowers stroke risk, evidence supporting the practice has been limited.
LAAOS III, conducted at 105 centers in 27 countries, was designed to provide a definitive answer. When the trial was stopped based on a recommendation from the data and safety monitoring board after a second planned interim analysis, it had randomized 2,379 patients to LAA occlusion and 2,391 to no occlusion during cardiac surgery. Trial participants had AF, a CHA2DS2-VASc score of at least 2, and an indication for cardiac surgery. Overall, mean patient age was 71 and mean CHA2DS2-VASc score was 4.2; two-thirds of the participants were men.
Cardiac surgery involved a valve procedure in around two-thirds of patients, with about 20% undergoing isolated CABG and 23% isolated valve surgery. Roughly one-third received AF ablation. Mean bypass time was significantly longer in the occlusion group (119 vs 113 min), as was mean cross clamp time (86 vs 82 min; P < 0.001 for both).
Several LAA occlusion techniques were permitted in the trial, although the preferred approach was amputation of the appendage followed by suture closure of the stump, Whitlock said.
At discharge, more than 80% of patients in each group were on oral anticoagulation, and at 3 years, that proportion had declined slightly to 76.8% overall.
On that background, surgical occlusion of the LAA reduced the rate of ischemic stroke/systemic embolism over an average follow-up of 3.8 years, driven by a lower rate of ischemic stroke (4.2% vs 6.6%; HR 0.62; 95% CI 0.48-0.80). Although the curves started to diverge just days after surgery, a landmark analysis showed that the advantage for LAA occlusion was greater after 30 days (HR 0.58; 95% CI 0.42-0.80).
There were no significant differences in systemic embolism (0.3% in each arm), death (22.6% vs 22.5%), or hospitalization for heart failure (7.7% vs 6.8%) during follow-up, or in bleeding, heart failure, or death in the perioperative period. Hospitalization for heart failure was the primary safety outcome because prior studies had shown there might be an issue stemming from a drop in atrial natriuretic peptide levels after occlusion. That concern was not borne out here.
“So we have a safe, effective intervention that can be done at low cost, low risk for patients,” Whitlock said.
Oral Anticoagulation Remains Important
Whitlock pointed out that even though warfarin and the direct oral anticoagulants are highly effective at preventing strokes in patients with AF, there is still a residual risk, and LAAOS III shows that this can be reduced with surgical LAA occlusion.
It behooves the percutaneous devices to follow on the heels of LAAOS III and examine this as an additive procedure for patients who are at particularly high risk of stroke. Richard Whitlock
The trial does not support eliminating oral anticoagulation, however. “If you want to have the greatest reduction in your stroke risk, then it’s the additive effect of these two interventions,” Whitlock said. He noted, however, that a subgroup analysis indicated that patients who were not on oral anticoagulation at baseline still appeared to derive a benefit from surgical elimination of the appendage. So if a patient has a contraindication to long-term anticoagulation, he said, “at least you can rest assured that there still is some stroke protection, but it’s not as good as if you’re on your oral anticoagulant and you have the left atrial appendage occluded.”
Williams said it would be interesting to dig further into this question of whether anticoagulation management could change after the LAA is surgically occluded. Another subanalysis that would be worth doing, he added, is one focused on how the method of occlusion—which could affect factors like bleeding risk and cost—influences the results.
Asked about potential implications related to use of transcatheter LAA occlusion devices, Whitlock stressed that the LAAOS III results apply to surgical occlusion during operations for other reasons only. “This is the first proof that left atrial appendage management reduces the risk of ischemic stroke, but it’s in these patients and with this approach,” he said. “I think it behooves the percutaneous devices to follow on the heels of LAAOS III and examine this as an additive procedure for patients who are at particularly high risk of stroke.”
To that point, Richard Page, MD (University of Vermont, Burlington), writes in an accompanying editorial that the benefits seen in LAAOS III on top of oral anticoagulation “raise the question of whether percutaneous occlusion, when added to ongoing oral anticoagulation, might provide benefit over anticoagulation alone in high-risk patients with atrial fibrillation. This intriguing question would require careful study, given the added cost and risk of that invasive procedure.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Whitlock RP, Belley-Cote EP, Paparella D, et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med. 2021;Epub ahead of print.
Page RL. The closing argument for surgical left atrial appendage occlusion. N Engl J Med. 2021;Epub ahead of print.
Disclosures
- LAAOS III was funded by the Canadian Institutes of Health Research and others.
- Whitlock reports grants from Bayer and Roche; grants and personal fees from Boehringer Ingelheim; and personal fees from AtriCure and PhaseBio unrelated to the study.
- Page reports no relevant conflicts of interest.


Comments