PERFORMANCE II: Low Stroke Rate Seen With All-Inclusive Carotid Stent
The recently approved device simplifies operator manipulations by loading a balloon and filter onto the stent.
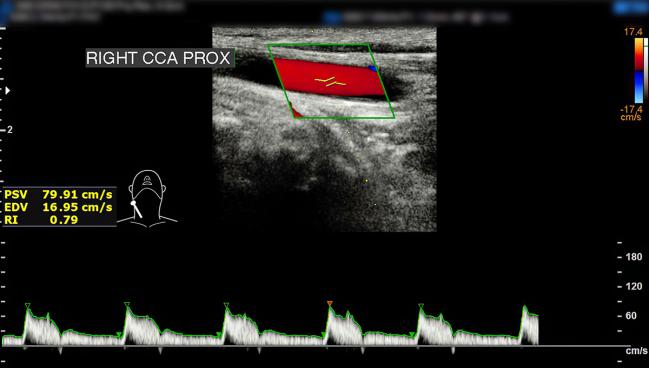
A “three-in-one stent” for carotid artery revascularization that includes both an embolic filter and a postdilation balloon appears to offer one of the lowest stroke rates ever reported in patients at high risk for surgery, according to data from the multicenter PERFORMANCE II trial.
The combined rate of any stroke at 30 days postprocedure, plus ipsilateral stroke between 31 days and 12 months, was 1.8% in patients in the single-arm trial testing the Neuroguard Integrated Embolic Protection (IEP) carotid stent system (Contego Medical).
To TCTMD, lead author William A. Gray, MD (Lankenau Heart Institute, Wynnewood, PA), said that low rate combined with the absence of any major stroke out to 2 years puts stroke protection with this device on par with what has been seen with other stroke preventative therapies like left atrial appendage occlusion and patent foramen ovale closure.
“This is the first new carotid stent in more than a decade. Because of that, we needed to look at not only safety, but also durability and restenosis,” he said.
The rate of target lesion revascularization was 1.1%, with no instances of clinically driven TLR.
In October 2024, the US Food and Drug Administration approved Neuroguard on the basis of data from the PERFORMANCE and PERFORMANCE II trials. The approval came approximately 1 year after Medicare’s National Coverage Determination expanded access in the United States to carotid artery stenting (CAS).
In an editorial accompanying the paper in JACC: Cardiovascular Interventions, Bernardo Cortese, MD (Harrington Heart and Vascular Institute, Cleveland, OH), and colleagues say as far as they can tell, the 30-day rate of all stroke plus ipsilateral stroke through 12 months “is the lowest ever reported in a similar multicenter trial, and theoretically, this device has responded well to the question of whether it is possible to reduce the risk for periprocedural ipsilateral stroke during CAS interventions.”
Still, Cortese and colleagues say questions remain about the reproducibility of the results, especially in light of contemporary registry data showing similarly low rates of 30-day ischemic stroke with carotid endarterectomy (CEA) in a population not unlike PERFORMANCE II.
“Additionally, CAS performed at centers with > 100 procedures in the past 5 years was found to be a predictor of protection against the primary composite endpoint of death and stroke at 30 days,” they write. “Such data are not derived from randomized controlled trials and thus should be evaluated with caution, but they reflect the importance of operator experience in reducing the risk for major events.”
PERFORMANCE II Results
The researchers enrolled 305 mostly asymptomatic high-surgical-risk patients (mean age 69.6 years; 34% women) from 32 centers in the United States and Europe. More than 40% of patients had diabetes, more than 90% had hyperlipidemia and hypertension, 10.5% had prior CEA, 19% had a prior TIA, and 21.3% had a history of stroke.
The mean lesion length was 19.1 mm, mean carotid stenosis severity was 73.7%, and 34.5% of lesions were severely calcified. Patients were classified as high risk based on physiological comorbidities (47.2%), anatomical conditions (24.3%), or both (28.5%). Lesion predilation prior to insertion of the carotid stent system was performed in 63.9%.
Procedural success was achieved in 96.7% of cases. The primary study endpoint, defined as death, all stroke, and MI within 30 days of the index carotid stenting procedure plus ipsilateral stroke from 31 days through 12 months postprocedure, was 2.8%. One patient died of cardiac causes on day 30, resulting in a stroke/death rate of 1.6% and a stroke/death/MI rate of 2.3%.
According to Gray, the three-in-one aspect lends itself to new or early CAS operators because it cuts down on some of the technical work by having everything the operator needs loaded onto a single device, making it easy to deploy the stent and balloon and quickly retrieve the filters.
“That’s as opposed to multiple steps of wire and catheter exchanges that you would do with a device that doesn’t have everything on it,” he added. “This cuts down on the manipulations you would otherwise be doing and it’s just much simpler and safer.”
But Cortese and colleagues say they are not convinced the study completely clarifies the performance and potential of the device, noting that they would like to see diffusion-weighted imaging (DWI) as well as direct comparisons of Neuroguard with other carotid stent systems and with CEA.
In response, Gray told TCTMD that early feasibility studies did employ DWI and showed a reduction in new DWI abnormalities following the procedure “along the same lines of what you see in endarterectomy and in [transcarotid artery revascularization]. So, that work had been done prior to the pivotal trial, and it provided more incentive to proceed with the pivotal trial of this device in this patient population.”
As for individual head-to-head comparison trials, Gray said they are likely unrealistic given the expense and time they would take for a device that is already FDA approved, and in light of the ongoing CREST 2 trial of CEA or CAS with a filter versus intense medical management in asymptomatic patients.
In his own practice, he expects Neuroguard to be a “workhorse” device for carotid stenting procedures.
“I say that because in this trial we had a very low number of patients who didn’t qualify based on landing area or tortuosity,” Gray noted. “I really believe this is going to be applicable to the majority of carotid stent patients we approach. It reduces time of procedure, which has been shown to be associated with better outcomes, and so there's just no reason not to use it.”
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Gray WA, Metzger DC, Zidar J, et al. The PERFORMANCE II trial a prospective multicenter investigation of a novel carotid stent system. JACC Cardiovasc Interv. 2025;18:367-376.
Cortese B, Frazzetto M, Shishehbor MH. All-in-one integrated device for carotid stenting: the last mile to attain surgical revascularization? JACC Cardiovasc Interv. 2025;18:377-379.
Disclosures
- Gray reports having served as a consultant for Contego Medical, Cordis, Boston Scientific, InspireMD, and Medtronic; and has received institutional research support from Contego Medical.
- Cortese reports no relevant conflicts of interest.




Comments