PET May Be Able to Pinpoint PAD Patients at Risk of Restenosis
Tracers that indicate inflammation and calcification prior to angioplasty are linked to 1-year restenosis, a small study shows.
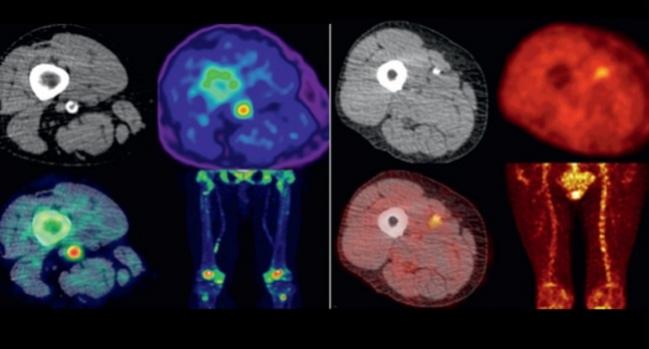
Signs of inflammation and calcification on positron emission tomography (PET) in patients with symptomatic peripheral artery disease prior to percutaneous transluminal angioplasty (PTA) are linked to 12-month restenosis risk, early data from a small study show.
The findings were published online yesterday in JACC: Cardiovascular Imaging.
“For the first time, we describe a method of identifying complex metabolically active plaques and patients at risk of restenosis that has the potential to select patients for intervention and to serve as a biomarker to test novel interventions to prevent restenosis,” Mohammed M. Chowdhury, MBChB (Addenbrooke’s Hospital, Cambridge, England), and colleagues write in their paper.
Constantino Peña, MD (Miami Cardiac & Vascular Institute, FL), commenting on the study for TCTMD, said this method—if it bears out—would fill an unmet clinical need.
“Right now we group patients all together. When we look at risk factors for restenosis, we see that maybe they have a longer lesion or a calcified lesion. We look at the morphology of the lesion. We may look at patient factors, such as if they’re smoking or are diabetic. However, we have no way to quantify what their preprocedure risk of restenosis is,” he observed.
The study is thought-provoking, Peña said. “If we have the ability to identify and stratify a patient’s chance of restenosis prior to treating them, can we then modulate our therapy in order to get better results for that particular patients? At the same time, is there a way that we can now evaluate [new] therapies?”
Two PET Tracers
The investigators prospectively enrolled 50 patients with unilateral symptoms of intermittent claudication or critical limb ischemia who were slated to undergo superficial femoral artery (SFA) interventions. All underwent PET scans employing 18F-fluorodeoxyglucose (18F-FDG) and 18F-sodium fluoride (18F-NaF) tracers; the images were co-registered with computed tomography (GE Discovery 690; GE Healthcare) to map inflammation and microcalcification, respectively, in the SFA before and 6 weeks after PTA.
Ultimately 40 patients completed the study protocol. Reasons for withdrawal included stenting subsequent to suboptimal PTA (n = 5), lack of 6-week follow-up scan (n = 3), and angioplasty not being performed due to unfavorable lesion profile (n = 2).
Baseline characteristics were similar in the patients who did and did not experience restenosis by 12 months. In the restenosis group, PET imaging showed significantly greater inflammation and microcalcification—expressed as median target-to-background ratio maximum (TBRmax)—before PTA.
PET Imaging: Median TBRmax Before PTA
|
|
Restenosis (n = 14) |
No Restenosis (n = 26) |
P Value |
|
18F-FDG |
2.43 |
1.63 |
< 0.001 |
|
18F-NaF |
2.61 |
1.69 |
< 0.001 |
The difference was also apparent at 6 weeks, by which time the values for patients without restenosis had decreased compared with baseline. That decrease in tracer activity may be due to positive remodeling after angioplasty, the researchers suggest.
What Next?
Would PET/CT imaging be practical in the clinical setting? The investigators say yes: “Given the increasing ease of access to PET imaging in many healthcare systems, and the simplicity and low cost of the radiotracers used, our study opens up the possibility of routine PET imaging in patients with PAD prior to intervention.” A single PET/CT exam costs approximately £550 to £800 in the United Kingdom, they point out, whereas reintervention costs approximately £770 to £800 beyond the initial cost of PTA.
Still, the authors caution that future studies must test the abilities of PET in larger cohorts to see whether the imaging can improve patient management in the setting of symptomatic PAD.
For Peña, an interventional radiologist, the biggest obstacles to adoption would likely be cost and the availability of the technology. “Even though there’s been increased access to PET imaging, I still think that with obtaining these scans there still may be some initial barriers,” such as less experience among imagers with 18F-NaF, he said. Even so, Peña added, “I think the science makes sense.”
He pointed out that 90% of patients in the study were on statins. “One of the major things we do with medical management is a statin. [This suggests] there’s more to it that just that,” Peña explained.
In terms of how the PET findings could improve care, Peña explained that this potentially could happen upfront (systemic therapies to reduce inflammation or more aggressive medical management to reduce restenosis risk), during the procedure (aggressiveness of intervention), or posttreatment (close monitoring).
Sanjay Divakaran, MD, Piotr S. Sobieszczyk, MD, and Marcelo F. Di Carli, MD (all Brigham and Women’s Hospital, Boston, MA), point out in an editorial that patients here received only angioplasty, not stenting. They note that stenting is standard of care for lesions longer than 100 mm or, as was the case for 10% of the original 50 patients, if PTA yields suboptimal results. Additionally, the study excluded diabetic patients on insulin, common among the PAD population. Nor does the paper report on any changes in medical therapy or lifestyle after treatment that might have influenced restenosis.
“Despite these limitations, this is an important proof-of-concept and hypothesis-generating study regarding the potential of targeted vascular PET imaging in the management of patients with PAD, particularly in an era when the power of PET imaging in cardiovascular disease continues to grow,” the editorialists agree.
And while PET’s use in patient management is indeed promising, they conclude, “perhaps even more importantly, further studies using PET may teach us more about the pathophysiology of PAD and improve both our diagnostic and therapeutic capabilities for a disease that is associated with significant cardiovascular morbidity and mortality.”
Photo Credit: JACC: Cardiovascular Imaging. Central Illustration (adapted).
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Chowdhury MM, Tarkin JM, Albaghdadi MS, et al. Vascular positron emission tomography and restenosis in symptomatic peripheral arterial disease: a prospective clinical study. J Am Coll Cardiol Img. 2019;Epub ahead of print.
Divakaran S, Sobieszczyk PS, Di Carli MF. The potential of PET in the management of peripheral arterial disease. J Am Coll Cardiol Img. 2019;Epub ahead of print.
Disclosures
- Chowdhury reports being funded by fellowships from the Royal College of Surgeons of England and the British Heart Foundation.
- Divakaran reports being supported by a T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute.
- Di Carli reports being supported by the National Heart, Lung, and Blood Institute and having received consulting fees from Sanofi and General Electric, research grants from Spectrum Dynamics and Gilead Sciences, and honoraria from GE Healthcare.
- Sobieszczyk and Peña report no relevant conflicts of interest.


Comments