Plant-Derived Compound Shows Early Promise for Small AAAs
In aneurysms normally chosen for surveillance, the endovascular technique slowed growth by 70% in the small phase I trial.
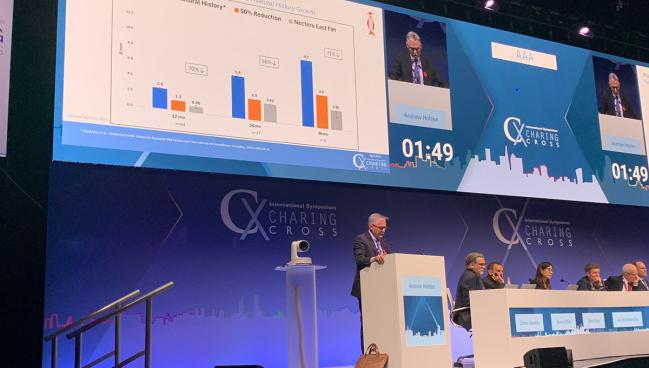
LONDON, England—A compound derived from tree bark and used as part of an endovascular procedure may offer patients with small abdominal aortic aneurysms (AAAs) an alternative to years of imaging surveillance.
Presenting phase I results here at the 2025 Charing Cross International Symposium, Andrew Holden, MBChB (Auckland Hospital, New Zealand), said that there is currently no effective way to manage the estimated 2 million patients who have small AAAs (3.5-5 cm).
The investigational technique with the compound has moved into phase II studies to see if it can fulfill the proof-of-concept of early clinical results, which suggest that when delivered directly to the aneurysm via a dual-balloon catheter system, it can slow and potentially stabilize the growth rate by essentially enhancing elastin that is lost with aging.
“Abdominal aortic aneurysms are manifest by a breakdown of elastin in the wall, causing the wall to weaken [and] bulge,” Holden said. The Nectero EAST system (Nectero Medical) uses pentagalloylglucose (PGG), which is derived from plant polyphenols, and has shown potential for strengthening weakened tissue, hindering enzymatic degradation, and reducing rupture risk.
The phase I trial was conducted at six centers in Hong Kong, Columbia, Latvia, New Zealand, and Australia. Among the 46 patients (mean age 70 years; 11% women), 70% experienced less diameter growth over 12 months than what would have been expected based on natural history comparisons (0.8 vs 2.6 mm; P < 0.001). Procedural success was 100%, with no major adverse events.
In a small group of patients with 3-year follow up, the diameter growth continues to be about 70% lower than anticipated by natural history (2.85 vs 9.7 mm) and this slowed growth appears to be larger for those with smaller aneurysms at baseline.
“It’s well tolerated by patients and takes an hour to do the procedure,” D. Christopher Metzger, MD (OhioHealth Riverside Methodist Hospital, Columbus), told TCTMD. “All of our patients have gone home the same day.”
Metzger’s center is one of 40 clinical sites that have been enrolling patients into a phase II/III study on the basis of the phase I data.
He described the procedure as “clever” and said if it makes it through the approval process, it could be a source of both physical and psychological relief for some patients.
“What I have found is that patients who have a 3.5- to 5-centimeter aneurysm don’t necessarily want to wait for it to get big enough for us to do something about it,” he said. “We can’t fix it in the traditional way that we fix abdominal aneurysms, nor should we, but people are more interested in this option, which is same-day outpatient in most cases, than I would’ve predicted that they would’ve been.”
On Pause for Now
In November 2023, the US Food and Drug Administration granted the treatment a breakthrough therapy designation followed by a fast-track designation a few months later. But just last month, the phase II/III study was placed on hold after the FDA raised concerns about acute and transient elevations in liver enzymes in a small number of patients.
This impacts all recruiting and consenting into the trial, said Holden, adding that some changes are being made to the study protocol. Those include enhancing exclusion criteria and increasing postprocedural monitoring.
“This seem to be a relatively isolated event in those predisposed to liver issues,” Metzger said. “I think we’ll probably see recommendations for excluding patients with preexisting liver disease or predisposition to liver disease. We have seen this issue before with things like statins where there were some early concerns about liver enzyme elevations, but the number of people affected was very low.”
During the discussion, Holden was asked whether there is a threshold at which the technique can no longer slow down the growth rate of the aneurysm and perhaps needs to be repeated.
“It’s a great question and it’s something we’re starting to address,” he noted. “There have been some patients . . . that initially had a response and then [the aneurysm] started to grow again.”
The question of whether to repeat the treatment will likely come from longer-term follow-up, he added.
Metzger said his main concern isn’t that the treatment doesn’t work, it’s that the investigators may have chosen the wrong endpoint for the phase II/III study that is comparing the endovascular therapy to surveillance. The event-driven primary endpoint is a composite of all AAA-related death, rupture, and repair (open surgical or EVAR), or clinical indication for repair.
“In this small group, the number of deaths related to aneurysm rupture is going to be low. So, you have a great drug that may not prevent that very low event rate,” he added. “The other part of the endpoint is conversion to repair and any number of things could happen, including a decision by the patient or doctor that EVAR is warranted.”
Even if the primary endpoint doesn’t reach clinical significance, Metzger said there is little doubt based on the phase I data that the prespecified secondary endpoint of growth over time in AAA diameter at 24 months will be statistically better than surveillance.
“I hope that’s enough to get it approved, but I can’t predict that one,” he added.
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Holden A. First-in-human study on a local stabilizing agent for small- to medium-sized AAA. Presented at: CX Symposium. April 24, 2025. London, England.



Comments