Pre-AVR Cardiac Damage in PARTNER Patients Linked to Poorer QoL at 1 Year
The damage argues for earlier intervention, but FDA reps remain cautious about expanding intervention indications.
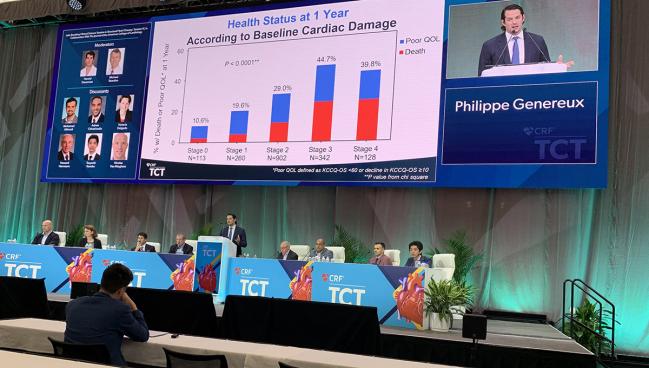
BOSTON, MA—Patients who undergo surgical or transcatheter aortic valve replacement (AVR) with evidence of cardiac damage at baseline have worse self-reported quality of life (QoL) a year later than those with no or lesser signs of damage, according to pooled data from the PARTNER 2 and 3 trials. Additionally, patients whose cardiac damage improved after AVR had greater improvement in QoL.
“We are demonstrating a clear relationship between the degree of cardiac damage and quality of life,” Philippe Généreux, MD (Morristown Medical Center, NJ), who presented the results here at TCT 2022, told TCTMD. “This points to the fact that we need to detect and treat earlier, before irreversible damage occurs, so we may improve long-term outcomes such as death and rehospitalization, but also quality of life after AVR.”
As Généreux showed in his presentation, each one-stage increase in baseline cardiac damage correlated with a 24% increase in the likelihood of a poor outcome at 1 year (P = 0.001). However, patients who improved their cardiac stage had a greater mean improvement in 1-year Kansas City Cardiomyopathy Questionnaire overall summary score (KCCQ-OS) compared with those with no change in cardiac stage or deterioration by one or more stages (P < 0.0001).
The data are part of a series of studies from the same investigators that attempt to outline the evolution of cardiac damage in aortic stenosis, Généreux told TCTMD. Initially, they described a novel staging classification that consists of stage 0: no extra-valvular damage; stage 1: left ventricular damage; stage 2: left atrial/mitral valve damage; stage 3: pulmonary artery/tricuspid valve damage; and stage 4: right ventricular damage. More recently, they showed that those with most-severe cardiac damage prior to AVR are more apt to die or be readmitted within 2 years, and that changes in the extent of the extravalvular damage at 1 year impact 2-year mortality risk.
“The big message is: the worse your heart is at baseline and at 1 year, the worse your quality of life will be,” Généreux said. The challenge, he added, is to identify cardiac damage that is capable of regressing after AVR from damage that is not. In some cases, he said, early intervention may be appropriate, while in others, more-aggressive secondary prevention may improve outcomes and QoL.
Making the Case for Staging
For the analysis, Généreux and colleagues pooled 1,974 patients (mean age 81 years; 45% women) with severe aortic stenosis who underwent AVR in the PARTNER 2A, 2B, and 3 trials and who had complete transthoracic echocardiography (TTE) data for staging at baseline. Of these, 40% underwent SAVR and 60% received TAVI. At baseline, 6.1% were stage 0, 14.5% were stage 1, 51.4% were stage 2, 20.9% were stage 3, and 7.1% were stage 4 on the cardiac damage classification system.
Patients at all stages of cardiac damage saw improvements in the KCCQ-OS score, but the extent of the improvement correlated with stage of damage, such that those in stages 3 and 4 saw the least improvement from baseline to 1 year and those in stage 0 or 1 saw the greatest improvements.
The question is: where along the continuum can you still reverse things? Michael Reardon
Events at 1 year for stage 0 through 4 ranged from 10.6% to nearly 40% for the composite of death, KCCQ-OS < 60, and a decline in KCCQ-OS of 10 greater. Similarly, the individual endpoint of death ranged from 2.5% to 21.4% for stage 0 and stage 4, respectively.
Of 1,120 patients with evaluable echocardiography data a year after AVR, cardiac damage improved in 15.6%, was unchanged in 57.9%, and worsened in 26.5%. Extent of cardiac damage at 1 year correlated with degree of KCCQ-OS improvement or worsening, with patients who improved their cardiac stage reporting a greater mean improvement compared with those who had either no change or deterioration by one stage or more (26.8 vs 21.4 vs 17.5 points; P < 0.0001).
Généreux said three ongoing trials in symptomatic and asymptomatic patients—EARLY TAVR, EVoLVeD, and PROGRESS—will provide more insight into the impact of early intervention prior to development of irreversible cardiac damage on prognosis.
In a press conference prior to the presentation, Robert Bonow, MD (Northwestern University Feinberg School of Medicine, Chicago, IL), said the concept of pre-AVR cardiac damage deserves further exploration.
“It's not surprising that the patients who have pulmonary hypertension and heart failure have worse outcomes, but those who have improvement—their RV function and pulmonary pressures come down—have a better outcome in terms of death and quality of life,” Bonow commented.
Late-breaking session moderator Michael J. Reardon, MD (Houston Methodist DeBakey Heart & Vascular Center, TX), said the cardiac damage concept helps clarify the emerging understanding of aortic stenosis as a continuum.
“The question is: where along the continuum can you still reverse things?” Reardon said. “Have you thought about going back to any of these stage 1, 2, 3, or 4 patients and [looking at] how many have already reached what you would think is irreversible fibrosis, that no matter what you do, they're not going to get better?”
“We don't have enough granularity to do that, but a lot of ongoing work with PROGRESS and TAVR UNLOAD and EVoLVeD will have more of what I call a multiparametric approach. . . . The story just starts with AS. We know how to treat it, but we don't really quite understand it,” Généreux said.
Preemptive TAVI, Indications, and the FDA
Généreux, the principal investigator for EARLY TAVR, has been a longtime champion of the theory that earlier intervention in asymptomatic AS has the potential to change the course of disease for many patients down the road. During an FDA Town Hall session at TCT 2022, he stressed that if a case can be made for earlier intervention in AS—specifically preemptive TAVI—it will be necessary to show why the things physicians can’t see in their AS patients may be as important as what they can.
Currently, for those with moderate AS, AVR is only indicated in patients undergoing cardiac surgery for other indications. Yet there are data, such as those from a large Australian registry, to suggest that 5-year mortality rates are more than 55% in those with moderate AS that is left untreated. Généreux’s own studies put the mortality rate at 30% at 2-and-a-half years, he said. Adding cardiac staging to AS severity grading, he noted, could make way for many of these patients to become candidates for either TAVI or SAVR earlier in their disease course.
Speaking in the Town Hall session, the FDA’s Changfu Wu, PhD, said the agency has purposely taken a cautious approach to TAVI indications by “chipping away at the apple gradually.” Likewise, his FDA colleague Kevin G. Soucy, PhD, said data spanning 10 years and beyond are needed to address concerns about durability before considering offering preemptive AVR therapy in moderate AS patients. Soucy added that patients in this situation will need to fully understand their options, including being told that the device could fail and require reintervention. “They need to understand what they are getting into and what might be in store for them,” he said.
There are so many things we have to think about and that’s why it’s important to have the surgical perspective with the interventionalist perspective and all try to make this decision together. Molly Szerlip
“Many of these patients will outlive their valves,” agreed Neal Kleiman, MD (Houston Methodist DeBakey Heart & Vascular Center), during the FDA Town Hall, “and we’re going to have to decide if the long-term cost of a second valve is worth it.” He added that for many patients it also may be confusing being told previously they didn’t need intervention, but that a procedure is now warranted.
Sammy Elmariah, MD, MPH (University of California, San Francisco), observed that physicians will “want to make absolutely certain the valve we are putting in is better than the valve that’s already been in.” But he said the fact that data such as Généreux’s are showing degrees of cardiac damage even in early-stage patients highlights the “crude methods” that physicians have been relying on to determine when the valve affects heart muscle enough to replace it.
“What we’re really getting at is performing these procedures at stages earlier in the disease course at which the injury to the muscle has been minimal, and hopefully prevent that irreversible injury from happening,” Elmariah said.
Among the things that still need to be considered thoughtfully in terms of preemptive AVR strategies are who truly is a moderate versus a severe AS patient and how many quality years they will gain from early intervention, noted Molly Szerlip, MD (Baylor Scott & White The Heart Hospital, Plano, TX), who was also part of the Town Hall discussion.
“There are so many things we have to think about and that’s why it’s important to have the surgical perspective with the interventionalist perspective and try to make this decision together,” she noted. “Just because we put in a TAVR or a SAVR doesn’t bring the patient back to someone who never had AS.”
Note: Généreux is a faculty member of the Cardiovascular Research Foundation, the publisher of TCTMD.
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Généreux P. Cardiac damage and quality of life after AVR: results from the PARTNER trials. Presented at: TCT 2022. September 18, 2022. Boston, MA.
Généreux P. The importance of pre-emptive TAVR in the future management of aortic stenosis. Presented at: TCT 2022. September 19, 2022. Boston, MA.
Disclosures
- Généreux reports serving as a consultant to Abbott Vascular, Abiomed, BioTrace Medical, Boston Scientific, CARANX Medical, Cardiovascular Systems Inc, Edwards Lifesciences, GE Healthcare, iRhythm Technologies, Medtronic, Opsens, Pi-Cardia, Puzzle Medical, Saranas, Shockwave, Siemens, Soundbite Medical Inc, Teleflex, and 4C Medical; serving as an advisor to Abbott Vascular, Abiomed, BioTrace Medical, Edwards Lifesciences, and Medtronic; receiving speaker fees from Abbott Vascular, Abiomed, BioTrace Medical, Edwards Lifesciences, Medtronic, and Shockwave; and receiving an institutional research grant from Edwards Lifesciences, for which he also is a proctor. He is a principal investigator for trials by Cardiovascular Systems Inc, Edwards Lifesciences, and 4C Medical. He holds equity in Pi-Cardia, Puzzle Medical, Saranas, and Soundbite Medical.




Comments