Rivaroxaban Helps Prevent More Events Than Aspirin Alone After Leg Interventions
The VOYAGER PAD subanalysis found an “astonishing” degree of vascular events and repeat procedures.
Following a lower limb revascularization, rivaroxaban (Xarelto; Bayer/Janssen) added to daily aspirin is better than aspirin alone for reducing the risk of first and total limb and CV events, a prespecified analysis from the VOYAGER PAD trial suggests.
Lead author Rupert Bauersachs, MD (Darmstadt Clinic, Germany), said the new analysis addresses an unmet need in the ongoing management of PAD patients who have already had at least one lower limb intervention, but remain at very high risk for serious adverse events and death.
“Rivaroxaban 2.5 mg twice daily effectively reduced first and subsequent limb and cardiovascular events, and [it] should be considered as an adjunctive treatment after lower extremity revascularization,” he said during a presentation at the recent American College of Cardiology 2021 Scientific Session. The study results were simultaneously published in the Journal of the American College of Cardiology.
These latest results add to what’s known from the main VOYAGER PAD study, which randomized lower-extremity revascularization patients from 34 countries to rivaroxaban or placebo in addition to daily aspirin. Rivaroxaban plus low-dose aspirin was superior to aspirin alone for the prevention of acute limb ischemia, a difference that was apparent as early as 3 months after randomization. Prior to that, the COMPASS trial had hinted at a benefit with the combination therapy in a large PAD-only population.
We've always known that these are high-risk patients, but I think exactly how high risk was not well understood until some of these data have come to light. Sahil Parikh
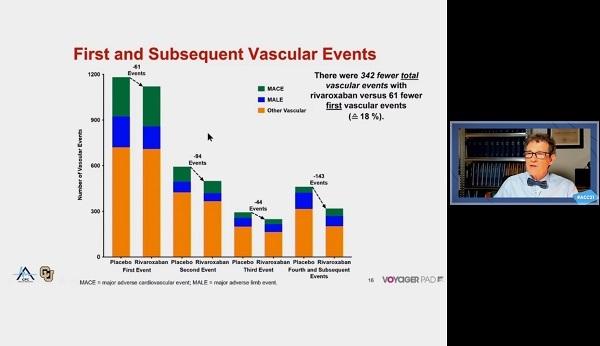
The new VOYAGER PAD analysis showed that at a median of 2.5 years of follow-up, there were 342 fewer total adverse events in the rivaroxaban group than in the placebo group, 61 fewer first vascular events, and 281 fewer subsequent vascular events beyond the first event.
“If we would have only looked at only first events, we would have missed more than 80% of the overall benefit,” Bauersachs observed.
Commenting on the study in a press conference, Sahil Parikh, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), called the magnitude of event rates “astonishing” and said the results support rivaroxaban and aspirin as a viable strategy to keep patients out of the hospital and protected from recurrent events.
“We've always known that these are high-risk patients, but I think exactly how high risk was not well understood until some of these data have come to light,” he said.
“I also think this trial continues to highlight the idea that the vascular bed that presents clinically is the one that will provide the patient with the most trouble, both in the acute and intermediate term,” Beckman noted. “So, a person who presents with stroke is much more likely to have stroke as their next event than a heart attack or a limb event.”
Limb Events Dominate
In the 6,564 VOYAGER PAD population, there were 4,714 total first and subsequent vascular events, including 1,614 primary endpoint events (acute limb ischemia, major amputation of a vascular cause, MI, ischemic stroke, or CV death) and 3,100 other vascular events. The most common type of vascular event was revascularization, which accounted for 64%, and the majority of those were in the index limb. MACE events were similar between groups.
Compared with aspirin alone, aspirin plus rivaroxaban total was associated with a significant reduction in primary endpoint events (HR 0.86; 95% CI 0.75-0.98) and in total vascular events (HR 0.86; 95% CI 0.79-0.95). Although a majority of patients did not have an event during the study period, 16.6% of the total study population did have more than one vascular event. Among second events, 60% occurred in patients who had a first peripheral revascularization event.
Even more robust differences in primary endpoint events and total events were seen in post hoc sensitivity analyses where rivaroxaban exposure was treated as a time-varying covariate: HR 0.42 (95% CI 0.37-0.49) for total primary endpoint events and HR 0.63 (95% CI 0.57-0.69) for total vascular events. Other analyses showed that rivaroxaban’s benefit when added to aspirin was consistent for both index and contralateral limb events, as well as for surgical and endovascular revascularization.
Unpacking Still to Do?
Given the high number of events, Beckman wondered if it is possible to determine if there are subgroups of patients who derive particular benefit from rivaroxaban, or who may even be harmed by it.
“I don't think it makes a lot of sense picking out groups with a very high risk, because these event rates are so high. Everybody is at a very high risk, and we need to do everything we can to reduce that risk,” Bauersachs said. “I think this applies to every patient undergoing a lower-extremity revascularization.”
Parikh noted that there are a number of things “buried deep in the tables” of the study that the VOYAGER PAD researchers will likely want to examine further, including the suggestion that patients who had atherectomy may have had more limb events. He also noted that it was curious that despite the reduction of acute limb events events, there was no change in vascular death.
“I think there's a great deal of information that we'll need to dig into further, and this will be fodder for much discussion in the field,” Parikh added.
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Bauersachs RM, Szarek M, Brodmann M, et al. Total ischemic event reduction with rivaroxaban after peripheral arterial revascularization in the VOYAGER PAD trial. J Am Coll Cardiol. 2021;Epub ahead of print.
Disclosures
- Beckman reports consultant fees/honoraria for Amgen, JanOne, Janssen Pharmaceuticals, and Sanofi; and serving on data safety monitoring boards for Bayer and Novartis.
- Parikh reports consultant fees/honoraria from Abbott, Boston Scientific, Cordis, Janssen, Medtronic, Penumbra, Philips, and Terumo; serving on data safety monitoring boards for Boston Scientific; research/research grants from Abbott, Shockwave Medical, Surmodics, and Trireme Medical; and serving on the speaker’s bureau for Inari.




Comments