SELECT: Semaglutide’s Impact on CVD Events Emerges Quickly
The benefit was seen as soon as 3 months. Investigators say there should be no delaying treatment for eligible patients.
MALAGA, Spain—Clinicians caring for patients with obesity or overweight and established cardiovascular disease should start patients on semaglutide (Wegovy; Novo Nordisk) as soon as possible.
That’s the message emerging from a new look at the SELECT trial, the large outcomes study that showed adding the glucagon-like peptide-1 (GLP-1) receptor agonist to usual care cuts the risk of major adverse cardiovascular events. In this latest analysis, the benefit was seen soon after starting therapy, possibly after just 3 weeks. By 3 months, there was a sustained, statistically significant difference in MACE favoring semaglutide.
“I think this is quite important in terms of overcoming clinical inertia among internists, among cardiologists,” said lead investigator Jorge Plutzky, MD (Brigham and Women’s Hospital, Boston, MA), during a presentation this week at the European Congress on Obesity. “They may not be able to wait until that next visit to begin pursuing therapies that could have a benefit on major cardiovascular events.”
Investigator Donna Ryan, MD (Pennington Biomedical Research Center, Baton Rouge, LA), who presented the data to the media, said that when the SELECT results were released in late 2023, one of the most intriguing findings was the separation of the Kaplan-Meier event curves. The benefit of semaglutide appeared to occur before patients achieved meaningful weight loss with treatment and even before they were titrated to the maximally tolerated dose.
Given these confirmatory data, Ryan said, there’s nothing to gain by waiting to prescribe semaglutide for eligible patients. “What this analysis says is: ‘Look, this treatment works early, so why wait?’” she told TCTMD.
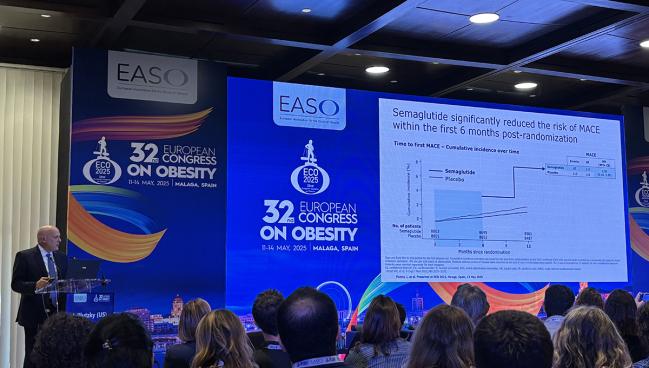
Benefit in the First 6 Months
The SELECT trial was conducted at 804 sites across 41 countries and included 17,604 patients 45 years or older who had preexisting CVD, a body mass index of at least 27 kg/m2, and no history of diabetes. Patients were randomized to once-weekly, subcutaneous semaglutide—uptitrated to a target dose of 2.4 mg—or placebo, both on top of standard CVD care that included lipid-lowering and antiplatelet therapy, among other agents.
Overall, semaglutide reduced the risk of cardiovascular death, nonfatal MI, and nonfatal stroke by 20% compared with placebo over a median follow-up of 40 months.
The new analysis focused on the first 6 months. Here, there were 67 major adverse cardiovascular events among those treated with semaglutide compared with 113 in patients randomized to placebo. This translated into a 41% lower relative risk of cardiovascular death, nonfatal MI, and nonfatal stroke in the semaglutide arm. The benefit was driven by 53% and 43% relative reductions in the risks of cardiovascular mortality and nonfatal MI, respectively.
“Keep in mind we’re drawing a circle here around just the people who have events within 6 months,” said Plutzky. These might represent the highest-risk patients enrolled in SELECT, but “you’re modifying the relative risk among the people who are going to have these early events.”
The first significant difference in major adverse cardiovascular events between treatment arms was seen at 20 days. By day 86, the difference in outcomes favoring semaglutide was statistically significant and remained so for the duration of follow-up. In the first 3 months, semaglutide reduced the risk of MACE by 38%; between months 3 and 6, the relative reduction in risk with semaglutide was 41%.
This is quite important in terms of overcoming clinical inertia among internists, among cardiologists. Jorge Plutzky
The researchers also performed an analysis to identify any potential baseline differences among patients who had a major cardiovascular event in the first 6 months. In this group, those who experienced MACE early were slightly older and had higher baseline high-sensitivity C-reactive protein (hs-CRP) levels when compared with those who had a clinical event beyond 12 months.
Researchers are uncertain what’s driving this early impact. “The fact that you can see benefits within 3 to 6 months points to other mechanisms that may be involved more rapidly than what we would typically look at in terms of weight change,” said Plutzky.
He suggested that it might relate to inflammation, noting that hs-CRP is reduced quite quickly after starting semaglutide.
Semaglutide did positively influence multiple cardiometabolic risk factors in the early part of the trial—including total cholesterol, LDL cholesterol, triglycerides, body weight, waist circumference, and systolic blood pressure—but the changes were modest. “Clinically, these are not changes we usually associate with major differences in cardiovascular events,” said Plutzky.
Ryan pointed out that the early reduction in major cardiovascular outcomes preceded clinically meaningful reductions in body weight, noting that by 3 months the reduction in body weight was less than 5%. “It’s even occurring before we get to the top dose [of semaglutide],” she said. “It takes a minimum of 16 weeks to the top dose, but we’re already seeing an effect very early on.”
Benefits Accrue Over Time
During the discussion following the presentation, Plutzky was asked about sticking with a 1-mg dose given the cost of medication and since the effect was seen before patients had been fully titrated to the 2.4-mg dose.
“I would not suggest to the patient that you got the 1-mg dose, you’re fine,” he replied. “Just as I would not suggest to a patient: ‘You got to 3 or 6 months, we’ve got all our benefit, let’s go home.’ That’s not the case, and that’s the risk of misinterpreting this [analysis].”
Benefits were evident early, but they also continue to accrue over time, said Plutzky.
On the basis of SELECT, the US Food and Drug Administration expanded the indication for semaglutide to include a reduction in cardiovascular disease events in patients with established disease and obesity/overweight. The Centers for Medicare & Medicaid Services also agreed to reimburse semaglutide when used to lower the risk of cardiovascular events in SELECT-like patients.
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Plutzky J, on behalf of the SELECT investigators. Early clinical benefit of semaglutide in adults with overweight or obesity and cardiovascular disease: a secondary analysis of the SELECT trial. Presented at: European Congress on Obesity. May 13, 2025. Malaga, Spain.
Disclosures
- Ryan reports consulting honoraria from Abbvie, Altimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Biohaven, Calibrate, Carmot/Roche, CinRx, Currax, eMed, Epitomee, Fractyl, Gila, Lilly, Nestle, Novo Nordisk, Pfizer, Source Bio, Structural Therapeutics, and Wondr Health.
- Plutzky reports consulting fees from Altimmune, Amgen, Boehringer Ingelheim, Esperion Therapeutics, Merck, MJH Life Sciences, Novartis, and Novo Nordisk, as well as institutional grants from Boehringer Ingelheim and Novartis.





Comments