The Skinny on Ultrathin DES: Meta-analysis Shows Some Long-term Advantages
Longer follow-up is needed, but the RCT data suggest a potential for ultrathin devices to reduce revascularization.

Compared with second-generation DES, devices with ultrathin struts are associated with less TLF, driven by reductions in clinically driven TLR, a long-term meta-analysis of multiple RCTs suggests.
The thinner struts have been thought to offer advantages in terms of producing less inflammation, vessel injury, neointimal proliferation, and thrombus formation. The new meta-analysis builds on the findings of a prior meta-analysis showing that ultrathin-strut DES were associated with a lower risk of TLF (composite of cardiac death, MI, or clinically driven TLR) at 1 year compared with conventional second-generation thin-strut DES.
Several additional large-scale RCTs that have been completed in recent years, with extended follow-up to a mean of 2.5 years, differentiate the newer meta-analysis, which was presented today by Yousif Ahmad, MD (Cedars-Sinai Medical Center, Los Angeles, CA), at the EuroPCR 2021 virtual meeting. It was also simultaneously published in the European Heart Journal. He told TCTMD that the new meta-analysis captures the totality of the available ultrathin data to the longest time point possible, important when trying to understand what the differences mean for operators and patients.
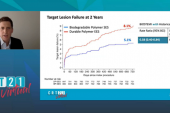
“We know that even with contemporary DES, which have excellent outcomes, we still get significant events occurring, particularly after the 1-year time point,” he explained. “In this meta-analysis we observed that target lesion revascularization was reduced by about 25% and target vessel failure by about 16% at 2.5 years. None of the other endpoints saw a significant difference between the conventional DES and the ultrathin ones, although we did see numerically less stent thrombosis with ultrathin-strut DES and also numerically fewer MIs, particularly target-vessel MIs.”
Commenting on the findings for TCTMD, David Kandzari, MD (Piedmont Heart Institute, Atlanta, GA), said there has been a progression of understanding through these meta-analyses, even though strut thickness as a single variable may be a bit of an oversimplification.
The theme that is emerging is one of superior benefit with ultrathin-strut drug-eluting stents. David Kandzari
“Differences in endpoints that are observed, and the timing of them, seem to vary across both the individual trials and the meta-analyses, but the theme that is emerging is one of superior benefit with ultrathin-strut drug-eluting stents,” he said. “We sometimes are overly focused on one element, but actually these may be instead interrelated. That is the drug, the polymer, and the stent design itself—each of these individual elements may contribute an additional advantage that, in aggregate, results in a clinically meaningful advancement.”
Puzzling and Inconsistent Mortality Signal
Ahmad and colleagues, led by Mahesh V. Madhavan, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), analyzed data on 16 RCTs comparing ultrathin-strut DES to conventional second-generation thin-strut DES for the treatment of CAD. In all, there were 20,701 patients randomized to receive an ultrathin-strut stent that had a strut thickness ≤ 70 µm or a conventional second-generation thin-strut DES with a strut thickness > 70 µm.
The ultrathin stents were Orsiro (Biotronik), MiStent (Micell), BioMime (Meril), and Supraflex (SMT). The control stents were Xience (Abbott), Resolute (Medtronic), Nobori (Terumo), BioFreedom (Biosensors), and Endeavor (Medtronic).
Compared with second-generation DES, the relative risk of TLF was 0.85 (95% CI 0.76-0.96) with ultrathin-strut DES, with the risk reduction similar for both early (≤ 1 year) and late (> 1 year) events. Similarly, ultrathin-strut DES were associated with a relative risk of TVF of 0.85 (95% CI 0.75-0.96), again with similar reductions for both early and late events. For repeat revascularization, the relative risk was 0.75 (95% CI 0.62-0.92) with ultrathin DES, although only the reduction in later events was statistically significant (RR 0.82; 95% CI 0.70-0.96). However, the relative risk reductions between the stent types for clinically driven TLR were consistent both up to 1 year (RR 0.84; 95% CI 0.74-0.95) and after 1 year (RR 0.86; 95% CI 0.76–0.98).
While there were no significant differences between stent types for the outcomes of MI and stent thrombosis, there was increased mortality seen with the ultrathin devices that did not reach statistical significance overall (RR 1.11; 95% CI 0.98-1.26)—the difference was, however, significant for early events (RR 1.25; 95% CI 1.04–1.51).
Ahmad said that while the mortality difference isn’t necessarily concerning, it is worth looking at further.
“It's difficult to explain mechanistically based on the data we have. It's slightly puzzling why a therapy which gives you significantly less revascularization, and a trend towards less stent thrombosis and MI, would then lead to an increase in mortality,” he noted. Something that may be of importance in looking at it further are patient-level data, although sensitivity analyses showed that all-cause death was significantly increased with the Orsiro stent in the BIOSCIENCE trial at 5-year follow-up, driven by noncardiovascular-related events.
Kandzari agreed that patient-level data would add a greater level of granularity to the issue, but said a trend toward higher mortality risk would be inconsistent with the body of the ultrathin evidence to date.
“It is also an oversimplification, perhaps, to think that all ultrathin-strut DES are equal in their safety and efficacy, and whether they should truly be grouped together in a meta-analysis like this or not may be debated,” he said. “The same argument goes for thin-strut, but not ultrathin-strut, contemporary DES like the Xience or the Resolute stents that were included. Should they, too, be grouped as one in this comparison, or might they need to be considered individually?”
Clinical Implications
Ahmad said a main take-away of the meta-analysis is that there’s good potential for ultrathin DES to improve upon second-generation DES.
“We now have a new technology, which might have superior outcomes. So, I think it needs to be studied further,” he said. “But at the moment, the data is very, very encouraging.”
Kandzari agreed. “The results should remind us that we haven't plateaued in outcomes with drug-eluting stents, and that even modest differences are important when we consider the implications of myocardial infarction or stent thrombosis,” he said. “The implications of a repeat revascularization are not inconsequential either, especially when we consider data from the [National Cardiovascular Data Registry], for example, that 10% of all percutaneous revascularization is still related to in-stent stenosis. If we can lessen that even to a modest degree, that represents a huge public health impact.”
He added that while ultrathin devices face an uphill battle to become commonplace in interventional cardiology due to the enormous cost associated with bringing them to market, they are making inroads as comparators in some clinical trials like SORT-OUT IX and X, and are showing evidence of superiority outside of RCTs in registries like SCAAR, where “there seems to be a very distinctive trajectory of repeat revascularization that's lower with ultrathin-strut stents than with alternative stent designs.”
Note: Study co-authors Gregg W. Stone, MD, and Ori-Ben Yahuda, MD, are faculty members of the Cardiovascular Research Foundation, the publisher of TCTMD.
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Madhavan MV, Howard JP, Naqvi A, et al. Long-term follow-up after ultrathin vs. conventional 2nd-generation drug-eluting stents: a systematic review andmeta-analysis of randomized controlled trials. Eur Heart J. 2021;Epub ahead of print.
Disclosures
- Madhavan reports a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute to his institution.
- Ahmad reports no relevant conflicts of interest.
- Kandzari reports consulting honoraria from Medtronic and Cardiovascular Systems; and institutional research support from Medtronic, Abbott, Biotronic, Boston Scientific, and Cardiovascular Systems.




Comments