TEST - COAPT Quality-of-Life Analysis Points to Significant, Sustained Benefits in Health Status With MitraClip
These findings for patients with severe heart failure and functional mitral regurgitation may be as important, if not more so, than the primary outcome.

NEW ORLEANS, LA—In the COAPT trial, patients with symptomatic heart failure (HF) and severe/moderate-to-severe mitral regurgitation (MR) felt better, with higher quality of life (QoL) scores, if treated with the MitraClip on top of standard care rather than with standard therapy alone, new trial results show.
These findings, experts said here at the American College of Cardiology (ACC) 2019 Scientific Session, may actually be more important to patients than the much-touted survival benefit and reduced heart failure hospitalizations reported from COAPT last September.
“When you look at patients with HF, particularly with NYHA class III and IV heart failure, they may care more about their quality of life than their survival,” Suzanne Arnold, MD (Saint Luke’s Mid America Heart Institute, Kansas City, MO), told TCTMD. “There’s actually studies that have shown that when you take patients who are very symptomatic, that’s their primary reason for getting this treatment in the first place. Survival is great, but if you feel terrible, then what’s the point?”
All patients enrolled in COAPT filled out two health status questionnaires at baseline and at 1, 6, 12, and 24 months: the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the SF-36 Physical and Mental Summary Scores.
Survival is great, but if you feel terrible, then what’s the point? Suzanne Arnold
As Arnold showed here, the KCCQ overall summary score over 24 months was significantly improved in the MitraClip (Abbott) group as compared with standard care, spiking to a change in score of 15.9 at 1 month then attenuating slightly to 14.5 at 12 months and 12.8 at 24 months, a statistically significant difference for all time points (P < 0.001).
Putting that change in perspective, Arnold described this as a “moderately large” improvement, noting that a 5-point difference is considered the minimum clinical difference typically perceptible to patients. Putting it another way, a majority of patients moved from NYHA class III to class I or II, she said. “So that’s certainly noticeable.”
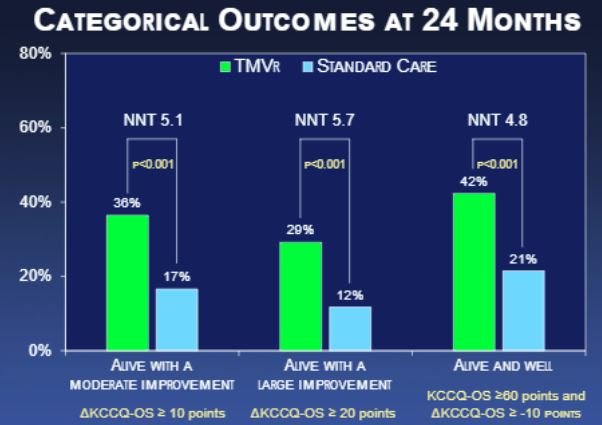
Additional KCCQ analyses showed significant and sustained differences in improvements in physical limitations, quality of life, total symptoms, and social limitations. As for the SF-36 questionnaire, here too MitraClip patients had significantly higher scores than patients receiving optimal medical care, with a delta of 5.3 at 1 month before dipping to 4.5 at 12 months and 3.6 at 24 months.
QoL benefits of MitraClip over standard care were sustained across a range of subgroup analyses, with the only signal of interest being an analysis by cardiomyopathy type. Patients with ischemic cardiomyopathy derived a larger benefit from MitraClip than did patients with nonischemic cardiomyopathy patients (a difference of 18 vs 8, although bother were statistically significant improvements). This, Arnold cautioned, “should be considered exploratory.”
Additional KCCQ analyses showed significant and sustained differences in improvements in physical limitations, quality of life, total symptoms, and social limitations. As for the SF-36 questionnaire, here too MitraClip patients had significantly higher scores than patients receiving optimal medical care, with a delta of 5.3 at 1 month before dipping to 4.5 at 12 months and 3.6 at 24 months.
Additional KCCQ analyses showed significant and sustained differences in improvements in physical limitations, quality of life, total symptoms, and social limitations. As for the SF-36 questionnaire, here too MitraClip patients had significantly higher scores than patients receiving optimal medical care, with a delta of 5.3 at 1 month before dipping to 4.5 at 12 months and 3.6 at 24 months.
QoL benefits of MitraClip over standard care were sustained across a range of subgroup analyses, with the only signal of interest being an analysis by cardiomyopathy type. Patients with ischemic cardiomyopathy derived a larger benefit from MitraClip than did patients with nonischemic cardiomyopathy patients (a difference of 18 vs 8, although bother were statistically significant improvements). This, Arnold cautioned, “should be considered exploratory.”
In additional analyses that accounted for the higher mortality in the standard-care arm (which could potentially bias the analysis in favor of surviving patients treated with the standard care), Arnold and colleagues found even larger benefits. In a Bayesian analysis, jointly modeling health status and mortality, the difference in the change in improvement on the KCCQ overall summary score was 18.5 at 1 month and 18.9 at 24 months. “So when we accounted for the different mortality rates between groups, we found that the health-status benefit was not only larger but also completely stable over time,” she explained to TCTMD.

But for two reasons, Arnold is convinced the effects are real. “One, the health status benefits are consistent with the main clinical results. If there was no improvement in heart failure hospitalization or survival, just a quality-of-life benefit, then I think you could say, well, maybe it’s placebo? But because everything went in the same direction, I think it’s reassuring
“The other thing is the magnitude of benefit and the amount that it was sustained,” she continued. “So you would expect a placebo effect to wane over time, and the fact that it didn’t makes it seem like it’s probably real.”
Following Arnold’s late-breaking clinical trial presentation, panelist Mayra Guerrero, MD (Mayo Clinic, Rochester, MN), put the data in frank perspective, saying: “This finding is as important if not more important than the primary endpoint.”
COAPT was unblinded, raising the possibility of a placebo effect in the intervention arm.
“That’s always a concern when you are looking at a patient-reported measures, and we’ve seen this before with angina: if you do something to somebody, or you give them a drug and say this is great, this is going to make you feel better,” she said.
But for two reasons, Arnold is convinced the effects are real. “One, the health status benefits are consistent with the main clinical results. If there was no improvement in heart failure hospitalization or survival, just a quality-of-life benefit, then I think you could say, well, maybe it’s placebo? But because everything went in the same direction, I think it’s reassuring
“The other thing is the magnitude of benefit and the amount that it was sustained,” she continued. “So you would expect a placebo effect to wane over time, and the fact that it didn’t makes it seem like it’s probably real.”
Following Arnold’s late-breaking clinical trial presentation, panelist Mayra Guerrero, MD (Mayo Clinic, Rochester, MN), put the data in frank perspective, saying: “This finding is as important if not more important than the primary endpoint.”
“The other thing is the magnitude of benefit and the amount that it was sustained,” she continued. “So you would expect a placebo effect to wane over time, and the fact that it didn’t makes it seem like it’s probably real.”
Following Arnold’s late-breaking clinical trial presentation, panelist Mayra Guerrero, MD (Mayo Clinic, Rochester, MN), put the data in frank perspective, saying: “This finding is as important if not more important than the primary endpoint.”
|
Related Coverage: "My Takeaways From TCT 2018: Sunglasses, Shadows, and the COAPT Eclipse" Health Status after Transcatheter Mitral- Valve Repair in Patients with Heart Failure |
“That’s always a concern when you are looking at a patient-reported measures, and we’ve seen this before with angina: if you do something to somebody, or you give them a drug and say this is great, this is going to make you feel better,” she said.
But for two reasons, Arnold is convinced the effects are real. “One, the health status benefits are consistent with the main clinical results. If there was no improvement in heart failure hospitalization or survival, just a quality-of-life benefit, then I think you could say, well, maybe it’s placebo? But because everything went in the same direction, I think it’s reassuring
“The other thing is the magnitude of benefit and the amount that it was sustained,” she continued. “So you would expect a placebo effect to wane over time, and the fact that it didn’t makes it seem like it’s probably real.”
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Suzanne V. Arnold SV, Chinnakondepalli KM, Spertus JA, et al. Health status after transcatheter mitral valve repair in patients with heart failure and secondary mitral regurgitation: COAPT trial. J Am Coll Cardiol. 2019;Epub ahead of print.
Disclosures
- Arnold reports having no personal conflicts of interest, other than an NIH/NHLBI grants, but notes that the COAPT trial was sponsored by Abbott and “designed collaboratively by the principal investigators and the sponsor,” although the health status analysis was conducted independently.


Comments