Trial of Cell Therapy for HF Misses Primary Endpoint, but Signals a Path Forward
DREAM-HF provides hints of an anti-inflammatory effect and benefits beyond the heart, setting up future trials.
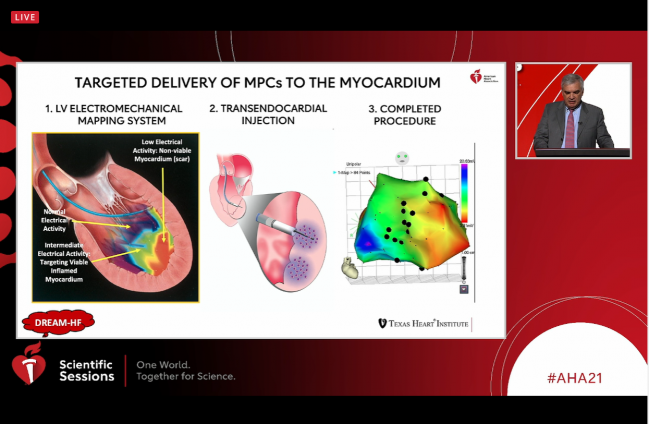
Targeted injection of mesenchymal precursor cells (MPCs) into the myocardium is safe but does not reduce recurrent nonfatal decompensated heart failure (HF) events in patients with chronic HF with reduced ejection fraction (HFrEF), the randomized DREAM-HF trial shows.
Through a mean follow-up of 30 months, the risk of that primary endpoint was no different between patients who received the cell therapy (rexlemestrocel-L; Mesoblast) and those who underwent a sham procedure (HR 1.2; 95% CI 0.8-1.7), Emerson Perin, MD, PhD (Texas Heart Institute, Houston), reported Sunday at the virtual American Heart Association (AHA) 2021 Scientific Sessions.
Key secondary endpoints—including terminal cardiac events and cardiac and all-cause death—occurred at similar rates in the two groups, as well. But there were suggestions of benefit when looking at other secondary outcomes (eg, nonfatal MI and nonfatal stroke) and in specific subgroups, such as patients with NYHA class II symptoms and those with elevated high-sensitivity C-reactive protein (hsCRP) levels at baseline, providing avenues for future research in a field that has been beset by underwhelming results.
“I think people should be excited about this,” Perin said at a media briefing. “This is the largest study in cell therapy that’s ever been performed and with a very long follow-up.”
Larry Allen, MD (University of Colorado, Aurora), who discussed the findings following Perin’s presentation during a late-breaking science session, viewed the results with a more-skeptical eye.
“Stem cells offer hope through a range of scientific approaches to potentially cure the underlying disease,” he said, but the “enthusiasm for stem cells in heart failure has been tempered in recent years with studies showing very small changes in left ventricular ejection fraction, a failure to demonstrate improved health outcomes in patients, and unfortunately, a fair amount of scandal.”
Designed on that background, DREAM-HF had some strengths, Allen said, pointing to the population that included clearly symptomatic patients with a mix of ischemic and nonischemic HFrEF; the “very large” size of the study, which included a sham procedure and blinding of the patients and HF investigators; and the assessment of clinically meaningful outcomes.
However, the primary endpoint and key secondary endpoints were neutral, with no trend toward benefit, he stressed. “The tertiary findings here are interesting, but [the] subgroups with significant benefit don’t necessarily make sense,” he said, pointing to the reductions in nonfatal MI and stroke without any effects on HF hospitalizations or other outcomes relevant to HF.
Moreover, though the cell therapy appeared to have a greater effect in patients with baseline inflammation, “prior studies of TNF inhibitors where this was the focus were not beneficial,” Allen pointed out.
But he ended with a more-positive message for the field at large: “Let’s keep up the investment. Heart failure remains a deadly disease,” he stated, adding that the stories around sacubitril/valsartan (Entresto; Novartis) and the sodium-glucose cotransporter 2 (SGLT2) inhibitors highlight that there’s still room to improve HF therapy.
“Failure of one approach does not condemn the entire field. Patients should understand that stem cells remain experimental. Study participation in these studies helps science. People should do it, but the current treatments are unlikely to help them individually,” Allen underscored, plugging the need to “double down on guideline-directed medical therapy.”
DREAM-HF
MPCs are allogeneic cells taken from adult human bone marrow that—when injected into the myocardium—are activated by cytokines produced by pro-inflammatory macrophages. The result of activation is a decrease in cardiac and systemic inflammation, a reduction in apoptosis, the induction of cardiac microvascular networks, and a reversal of endothelial dysfunction, Perin explained.
“Although this is a very attractive therapeutic target, previous trials of anti-cytokine therapy targeting TNF-alpha have failed,” he said.
Preclinical studies of the types of MPCs used in the current study have indicated that they have anti-inflammatory, immunomodulatory, and proangiogenic effects in models of ischemic and nonischemic cardiomyopathy, Perin said.
The first transendocardial injection of MPCs in a human patient was performed in 2006, and in 2015, Perin and colleagues published a phase II dosing study suggesting that the approach may reduce HF-associated events and adverse ventricular remodeling in patients with persistent HFrEF.
DREAM-HF, conducted at 51 sites in the United States and Canada, was designed to further explore the clinical impact of the approach. Investigators enrolled patients who had chronic HFrEF with NYHA class II or III symptoms, an LVEF of 40% or lower, and at least one high-risk enrichment criterion. All were receiving stable optimal medical therapy for at least a month before the study.
The main analysis included 537 patients (mean age 63 years; 80% men) who were randomized and treated. Most had ischemic cardiomyopathy (56%) and NYHA class III symptoms (62%). The mean LVEF was 29%.
The intervention, performed during a single procedure in the cath lab, involved transendocardial injection of 150 million MPCs guided by an LV electromechanical mapping system that directed the cells to areas of viable but inflamed myocardium. A sham procedure was used in the control arm.
The primary endpoint of the trial was not met, and Perin acknowledged that when that happens, “the rest of the findings are considered hypothesis-generating.” He added, however, that “this does not mean post hoc. I want to emphasize that all secondary endpoints that I will present were prespecified in the protocol and statistical analysis plan.”
Of note, the risk of nonfatal MI or nonfatal stroke was lower in the treatment (4.6% vs 13.0%; HR 0.35; 95% CI 0.18-0.66), with similar results in both NYHA class II and class III patients.
Cardiac death was no different between trial arms overall, although cell therapy was associated with a lower risk in patients with NYHA class II symptoms (8.0% vs 19.8%; HR 0.43; 95% CI 0.19-0.98), driven by reductions in patients who had elevated hsCRP levels (≥ 2 mg/L) at baseline.
A post hoc composite endpoint of cardiac death, nonfatal MI, or nonfatal stroke was reduced with the intervention both overall (20.3% vs 30.1%; HR 0.67; 95% CI 0.47-0.94) and in patients with elevated hsCRP (19.0% vs 32.4%; HR 0.55; 95% CI 0.35-0.88).
The intervention was shown to be safe, with no differences between groups in treatment-emergent or serious adverse events and no clinically meaningful immune-related responses in any patient, Perin reported. There were no complications related to LV angiography, although there was one LV perforation (0.4%) during LV mapping.
Both Local and Systemic Effects
The lack of an impact on the primary endpoint of recurrent nonfatal decompensated HF events indicates that “MPCs are not a decongestive therapy,” Perin said. “They work in a completely different way, directly in the myocardium.”
Once exposed to the inflammation within the heart, the MPCs are activated, producing cytokines and other products that have a beneficial effect in the myocardium and preventing cardiac death, Perin said, adding that the process also has a systemic effect that reduces risks of MI and stroke.
The approach studied here is an excellent example of personalized medicine, Perin stated. “We know where the benefits of the cells are, and that’s identified in the patients that have inflammation, so we know that if patients aren’t inflamed this therapy really isn’t going to help them,” he said. And once those patients are identified, the LV mapping system can be used to precisely deliver the MPCs to the areas of inflammation within the heart.
But more research is needed to get to the bottom of the mechanisms underlying any benefits, according to Biykem Bozkurt, MD, PhD (Baylor College of Medicine, DeBakey VA Medical Center, Houston TX), who commented on the results during a media briefing.
“There’s always a reason to do further research and understand the why, right? Because we are seeing a signal for a reduction in myocardial infarction and stroke, and that’s quite intriguing,” she said. “The question may need to be whether the control or the sham operation rates are higher than no operation and/or if the mesenchymal injection is resulting in microvasculature changes.”
Speaking to TCTMD, Terrence Yau, MD (University of Toronto, Canada), who was not involved in the study, provided a cautious interpretation of the results, particularly the large reductions in nonfatal MI, nonfatal stroke, and cardiac death (in the NYHA class II patients). “It’s just that they are moderately far down the list of secondary endpoints, so it’s hard to hang your hat on too much of that. It’s intriguing and it’s certainly fodder for how to design the next trial, but this isn’t quite what they had dreamt of several years ago when they designed the trial, obviously,” he commented.
Yau underscored the difficulty of conducting cell therapy trials, both because of the types of patients involved—with a lot of comorbidities—and because of the complexity of the treatments. It’s hard to know exactly how MPCs will react when injected into a particular patient, and that can make it difficult to choose the appropriate endpoints for clinical trials.
That said, the magnitude of the reductions seen in those secondary endpoints—which are much greater than those seen in trials of new drugs seen as major advances in HF—are large enough to warrant future MPC trials, Yau said. He pointed also to evidence regarding effects beyond the heart as one of the most interesting findings to come out of the trial.
Overall, Yau said, there are “lots of interesting things for discussion from this trial even if their primary outcome wasn’t positive.”
Perin said “the DREAM-HF trial turns a page on stem cells in heart failure because now we understand a clear mechanism of inflammation.” The cell therapy “addresses the core of what keeps heart failure going, which is a smoldering inflammation,” he said, “and when we see a decrease in stroke and MI, we see that the immune process—which is that of atherosclerosis and rupture of atherosclerotic plaque—is also impacted by the anti-inflammatory systemic effect of these cells.
“So actually we’re very excited because we have a lot to pursue now, and I think we’ll be very focused in exploring what we did see and not using an endpoint of . . . recurrent events of congestion, but an endpoint of mortality, stroke, and MI,” he predicted.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Perin EC. Randomized trial of targeted transendocardial delivery of mesenchymal precursor cells in high-risk chronic heart failure patients with reduced ejection fraction – the DREAM-HF trial. Presented at: AHA 2021. November 14, 2021.
Disclosures
- DREAM-HF was sponsored by Mesoblast.
- Perin reports consulting for Mesoblast.
- Allen reports consulting for Abbott, ACI Clinical, Amgen/Cytokinetics, Boston Scientific, Cytokinetics, and Novartis, and receiving research support from the National Institutes of Health/National Heart, Lung, and Blood Institute, the Patient-Centered Outcomes Research Institute, and the American Heart Association.
- Yau reports no relevant conflicts of interest.



Comments