Trimetazidine Safe but Not Protective Long-term After PCI
ATPCI investigators are “rather disappointed,” Roberto Ferrari said, that the antianginal’s metabolic effects didn’t bear fruit.
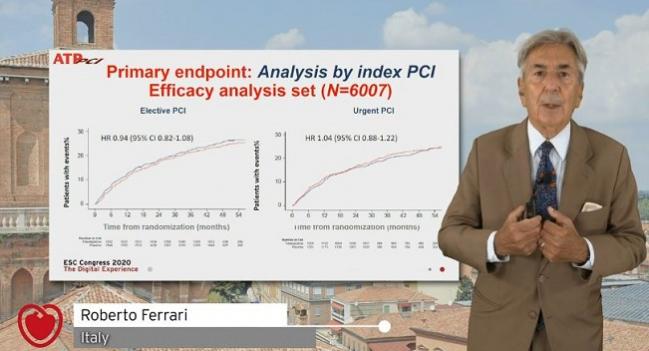
Trimetazidine on top of standard therapy among patients who’ve undergone PCI for stable angina or NSTE ACS doesn’t affect their long-term outcomes, the ATPCI trial has shown.
Participants in the randomized, placebo-controlled trial took trimetazidine at a dose of 35 mg twice daily for several years—to no avail, lead author Roberto Ferrari, MD, PhD (University of Ferrara, Italy), reported today in a Hot Line session of the virtual European Society of Cardiology Congress (ESC) 2020. The results were simultaneously published in the Lancet.
“Why trimetazidine? Because it is an antianginal agent different from the commonly used first- and second-line antianginal drugs, as it does not have any hemodynamic effect. Trimetazidine does not change heart rate, systolic or diastolic blood pressure, [or] pre- or afterload. Instead, it improves the metabolism of the ischemic myocardium,” he explained in his presentation.
However, as Ferrari told journalists ahead of ESC, this did not translate into benefit over a median follow-up of nearly 5 years.
“The take-home message from this study is that patients with stable angina and NSTEMI receiving optimized medical therapy, either antianginal or preventive, combined with successful PCI have a very low event rate and recurrent angina occurs only in a minority,” he said. “Also, unfortunately, improvement of cardiac metabolism with trimetazidine did not improve outcome or the occurrence of angina and is not necessary.”
Patients with chronic coronary syndrome “should consider themselves lucky,” Ferrari concluded, advising that it’s important for them to control their risk factors, take already-available antianginal drugs, and know that if symptoms do occur, angioplasty is indicated.
The ATPCI investigators were “rather disappointed about the results. We had really hoped the metabolic therapy could be beneficial,” he said in the press conference.
Asked by TCTMD whether trimetazidine deserves any further study, Ferrari suggested a higher dose might prove helpful. “In all honesty, I’ve been trying to test metabolic agents all my life . . . and I never managed to make them work in a chronic condition,” he said. “That is why I was hoping trimetazidine could be the one, but that was not the case—trimetazidine can be important to improve metabolism during the peak of exercise or during ischemia, but not in chronic conditions.
“So I am not so sure whether the idea of improving the metabolism will continue, I’m afraid,” Ferrari acknowledged.
Trimetazidine can be important to improve metabolism during the peak of exercise or during ischemia, but not in chronic conditions. Roberto Ferrari
ATPCI enrolled 6,007 patients (mean age 61 years; 77% men) after successful PCI at 365 centers in 27 countries, randomizing them to receive either oral trimetazidine 35 mg twice daily or placebo. Indications included stable angina, unstable angina, and NSTEMI, with 42% undergoing urgent PCI and the rest elective.
At baseline, nearly 100% were on antiplatelet agents, 96% were taking statins, and 93% were on some form of antianginal therapy.
The trial’s primary efficacy endpoint included cardiac death; hospital admission for a cardiac event; recurrence or persistence of angina requiring an addition, switch, or increase of the dose of at least one antianginal drug; or recurrence or persistence of angina requiring coronary angiography. Analyses were done based on intention-to-treat; around one in five patients in each group stopped their study drug early, with 9% doing so because of adverse events.
Median follow-up duration was 47.5 months, during which time the incidence of the primary endpoint was almost identical for the trimetazidine and placebo groups (23.3% vs 23.7%; P = 0.73). There also were no differences when it came to individual components or based on whether patients had undergone elective or urgent PCI.
Seventeen percent of patients—again, the same rate in both groups—experienced angina that led to angiography.
Why Didn’t It Work?
The researchers propose several reasons for trimetazidine’s lack of effect. “Much of this might be explained by the high routine use of antianginal medications after PCI. These medications might have been prescribed for reasons other than angina, such as blood pressure control, and therefore might have contributed to the absence of benefit seen with trimetazidine,” they write in their paper. “Additionally, ischemia might improve over time as collateral coronary flow develops, or when coronary plaques stabilize. Patients might also become accustomed to their condition and protect themselves from provoking angina.”
And for the ATPCI population in particular, they say, “combining successful PCI with optimal preventive and antianginal therapy was probably sufficient for symptom control in most cases.”
The trial, originally designed for 36 to 48 months of follow-up, had to be extended to 5 years due to low event rates. ATPCI investigators attribute this to the patient population—all had, by design, undergone successful PCI and patients tended to be young, with low atherosclerotic burden, preserved ejection fraction, and high use of preventive therapy. Alongside this, there have been improvements in PCI and disease management.
Discussant Stephan Windecker, MD (Bern University Hospital, Switzerland), agreed that angina is a worthy target for therapy. “One in four patients with chronic coronary syndrome suffer from angina,” he said, adding that angina is linked to worse quality of life, physical limitations, and poorer prognosis. The 2019 ESC guidelines list trimetazidine (class IIa recommendation) as a second-line therapy for patients with angina.
When it comes to long-term effects post-PCI, he described the ATPCI results as “conclusive.”
“Owing to the established efficacy of contemporary PCI for angina relief and lifestyle changes, antiplatelet therapy, ACE inhibitors, and potent lipid-lowering drugs for secondary prevention of ischemic events, trimetazidine has no additional therapeutic role in this patient population,” Windecker concluded.
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Ferrari R, Ford I, Fox K, et al. Efficacy and safety of trimetazidine after percutaneous coronary intervention (ATPCI): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;Epub ahead of print.
Disclosures
- Ferrari reports fees, honoraria, and travel expenses from Servier, research grants and personal fees from Novartis, and personal fees from Merck Serono, Boehringer Ingelheim, Sunpharma, Lupin, Doc Generici, Pfizer, and Spa Prodotti Antibiotici.


Waqar Ahmed