Ultrathin-Strut Orsiro Stent as Good as BioMatrix: BIODEGRADE
The trial included two stents with biodegradable polymers in an all-comer population and reported low event rates at 18 months.
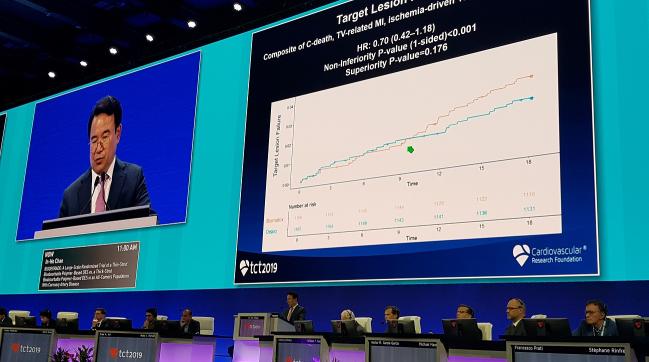
SAN FRANCISCO, CA—A head-to-head comparison of two coronary stents with biodegradable polymers in an all-comers population showed that the Orsiro ultrathin strut drug-eluting stent (Biotronik) was noninferior to the BioMatrix stent (Biosensors) with conventional strut thickness.
At 18 months, the rate of target lesion failure, a composite endpoint that included cardiovascular death, target vessel-related MI, and ischemia-driven target lesion revascularization, was 2.9% in patients treated with the BioMatrix stent and 2.1% in patients treated with Orsiro (P < 0.001 for noninferiority).
Presenting the results of the BIODEGRADE trial at TCT 2019, lead investigator In-Ho Chae, MD, PhD (Seoul National University/Bundang Hospital, Korea), said that the disappointment over the first fully bioresorbable Absorb coronary stent (Abbott Vascular) has led to a renewed interest in metallic DES with a biodegradable polymer. Even with the biodegradable polymer, Chae stressed, stent design is also important to achieving excellent outcomes.
“I believe that with strut thickness, [thinner] is better,” said Chae during the final late-breaking clinical trial session.
Session moderator Ajay Kirtane, MD (NewYork-Presbyterian/Columba University Irving Medical Center, New York, NY), noted that while the trial was a comparison of stents with different strut thicknesses—the Orsiro struts are just 60-80 µm compared with a strut thickness of 120 µm with BioMatrix—there are other differences. The polylactic acid polymer on the stainless steel BioMatrix stent degrades in approximately 9 months while the poly-L-lactic polymer on the cobalt-chromium Orsiro stent degrades over 12 to 24 months. Additionally, Orsiro utilizes sirolimus as its antiproliferative drug, while BioMatrix uses biolimus A9.
To date, there have been no head-to-head studies comparing Orsiro against the BioMatrix stent.
The LEADERS trial with BioMatrix showed the stent was superior to a conventional DES for the prevention of major adverse cardiac events and stent thrombosis, while the BIOFLOW II showed that Orsiro was noninferior to the everolimus-eluting Xience stent (Abbott Vascular) for the prevention of target lesion failure and stent thrombosis at 5 years. The BIOFLOW V study, which was presented 2 years ago at the European Society of Cardiology Congress, garnered significant attention when the 12-month data showed the Orsiro stent was superior to Xience, largely considered the best-in-class, for reducing the risk of target lesion failure at 12 months.
Low Event Rate in BIODEGRADE
The BIODEGRADE study included 2,341 patients recruited from 25 Korean hospitals scheduled for PCI. In total 1,175 patients were treated with the Orsiro stent and 1,166 treated with BioMatrix. The trial included a high proportion of patients with acute coronary syndrome, with 36.5% of patients presenting with unstable angina and roughly 10% of patients presenting with STEMI.
At 18 months, there was no significant difference in the rate of target lesion failure between the two stents. The curves began to diverge at approximately 1 year in favor of the Orsiro stent, but the difference did not reach significance. All clinical events—cardiac death, target vessel-related MI, ischemia-driven target lesion and target vessel revascularization, and stent thrombosis—were no different between the device arms. The patient-oriented endpoint, a composite of all-cause mortality, MI, and all repeat revascularization, also was similar between the stent arms.
In the subgroup analysis, there was a significant interaction by stent type in those with versus without diabetes. There was no significant difference in target lesion failure with Orsiro or BioMatrix in patients with diabetes, but for those without diabetes the rate of target lesion failure was 1.3% with Orsiro and 3.0% with BioMatrix (P = 0.024).
Yoshinobu Onuma, MD, PhD (Fujita Health University Hospital, Japan), one of the panelists, questioned the very low event rate in the trial. He noted that a previous meta-analysis showing a benefit of stents with thinner struts suggested the benefit emerges with a reduction in MI, but the rate of MI in BIODEGRADE was extremely low in both arms. In the present trial, there were no target-vessel MIs in the BioMatrix arm and just three MIs in the Orsiro-treated patients.
Chae explained they did not include periprocedural MIs in their endpoint, which may explain the lower event rate. Speaking during the session, Chae said that the overall clinical events observed in the trial was lower than expected. They assumed a 5% rate of target lesion failure with both stent types, but the actual event rate in both arms was less than 3.0%. While this might have been the result of underreporting, Chae stressed that the “study was done with the highest degree of scrutiny with periodic monitoring.” Just 0.6% of patients, for example, were lost to follow-up. Chae said that ethnic or genetic factors likely contributed to the lower event rate.
Michael Maeng, MD, PhD (Aarhus University Hospital, Denmark), also homed in on the very low event rate, noting that in the SORT-OUT VIII trial comparing BioMatrix against Synergy (Boston Scientific) the rate of target lesion failure was 4.4% and 4.0%, respectively.
Elvin Kedhi, MD, PhD (Isala Hartcentrum, Zwolle, the Netherlands), said that meticulous head-to-head comparisons of stent types typically involve data collection at multiple timepoints following the procedure to assess early enzymatic changes that reflect periprocedural MIs. “This probably explains why the event rates are so low,” said Kedhi.
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Chae I-H, on behalf of the BIODEGRADE investigators. Comparison of Biomatrix and Orsiro drug-eluting stents angiographic result in patients with all-comer coronary artery disease: a multicenter, randomized, open-label study (BIODEGRADE study). Presented at: TCT 2019. Sunday, September 29, 2019. San Francisco, CA.
Disclosures
- Chae reports no conflicts of interest.


Comments