After Failed Mitral Valve Surgery, Transcatheter Valve-in-Valve or Valve-in-Ring Shows Promise in High-Risk Patients
Treating patients with failed bioprosthetic valves yielded more predictable results and better outcomes than those with failed annuloplasty rings.
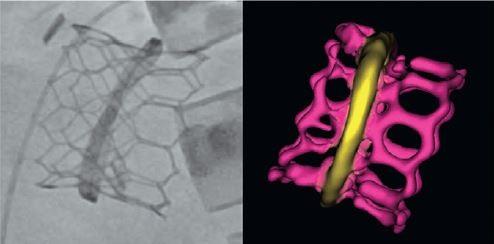
Among patients who experience failure of their bioprosthetic mitral valves or annuloplasty rings, and are at too high of a risk for another surgery, a secondary procedure to implant a transcatheter valve may be a better option.
Although structural interventionalists have been discussing and occasionally performing procedures like this for about the last decade, most patients ineligible for repeat surgery—either because of advanced age or comorbidity—were only managed medically, and not always successfully. But advances in technology and access have opened the door to a new era of treating these patients. In June, for instance, the US Food and Drug Administration’s (FDA) expanded the indication of the Sapien 3 transcatheter heart valve (Edwards Lifesciences), originally just used in the aortic position, to include valve-in-valve replacement for high-risk patients with failing bioprosthetic mitral valves.
“[Without] a surgical alternative, it was a bad situation for your patient . . . both from a mortality perspective and also a clinical perspective,” Eduardo J. DeMarchena, MD (University of Miami Health System, FL), who was not involved in the study, told TCTMD. But prior studies looking at valve-in-valve or valve-in-ring procedures in mitral disease have been limited by size, he added, himself having only performed about four of these procedures in the last several years.
Combined Experience
Bringing together the combined experience of operators at 25 centers worldwide, Sung-Han Yoon, MD (Cedars-Sinai Heart Institute, Los Angeles, CA), and colleagues reviewed outcomes in 248 patients who underwent transcatheter mitral valve replacement (TMVR) after a previous mitral valve surgery. Their findings appear in the August 29, 2017, issue of the Journal of the American College of Cardiology.
Most patients (71.0%) underwent the procedure after a failed bioprosthetic valve, while the remainder had failing annuloplasty rings. Mean STS score was 8.9 ± 6.8%, and more than half of the cohort was female (56.9%). Additionally, most patients were treated transapically (66.5%) and with balloon-expandable valves (89.9%).
Technical success was achieved in 92.3%, with lower technical success observed in the valve-in-ring cohort compared with the valve-in-valve patients (83.3% vs 96.0%; P = 0.001) due to more frequent need for a second valve. Reintervention was also more common in the valve-in-ring vs valve-in-valve population (16.7% vs 7.4%; P = 0.03).
While there were no differences between the valve-in-ring and valve-in-valve groups with regard to mitral valve mean gradient and mitral valve area, postprocedural LVEF was lower after valve-in-ring than valve-in-valve procedures (44.1 ± 15.4% vs 52.8 ± 12.0%; P < 0.001). Also, moderate or greater mitral regurgitation was higher postprocedure and at 30 days for the valve-in-ring group compared with valve in valve (postprocedure: 19.4% vs 6.8%; P = 0.003; 30 days: 13.6% vs 3.6%; P = 0.005).
At 30 days, there were no differences between the study groups in mortality, stroke, major bleeding, or major vascular complications, but patients in the valve-in-ring cohort had more frequent life-threatening or fatal bleeding (8.3% vs 2.3%; P = 0.03) and stage 2 or 3 acute kidney injury (11.1% vs 4.0%; P = 0.03). Hence, overall procedural success was lower after valve-in-ring compared with valve-in-valve procedures (58.3% vs 79.5%; P = 0.001).
Over a median follow-up of 220 days, a total of 48 patients died, bringing the cumulative event rate for all-cause mortality to 16.9% at 1 year. This was higher in the valve-in-ring than in the valve-in-valve group (28.7% vs 12.6%; P = 0.01). On multivariate analysis, both advanced age (HR 1.04; 95% CI 1.00-1.08) and having a failed annuloplasty ring (HR 2.70; 95% CI 1.34-5.43) were independently associated with all-cause mortality at 1 year.
‘Acceptable Outcomes’
“The challenges of the mitral [valve-in-ring] procedure may be attributable to several factors,” the authors explain. Annuloplasty rings are initially elliptical in shape and possess various degrees of rigidity so sizing can be difficult, they say. Also, the presence of native anterior mitral leaflets and insufficient fixation of the rings upon them can ultimately lead to the need for a second valve implantation or LVOT obstruction.
Even with these difficulties, the TMVR procedure provides “acceptable outcomes” for both patients with failed bioprosthetic valves as well as annuloplasty rings, Yoon and colleagues conclude.
In an editorial accompanying the study, John Webb, MD (St. Paul’s Hospital, Vancouver, Canada), Anson Cheung, MD, MSc (University of Washington School of Medicine, Seattle), and Danny Dvir, MD (University of Washington School of Medicine, Seattle), hypothesize that given its recent approval, the Sapien 3 device “will likely be the dominant [valve-in-valve] and [valve-in-ring] option in the United States for several years to come. However, it seems intuitive that the ability to reposition or remove an implant that is incorrectly positioned, sized, or obstructing the LVOT might be a desirable feature.”
A number of third-generation valves at various stages of development and commercialization have the ability to be retracted and repositioned. The ongoing development of mitral-specific transcatheter heart valves also “may play an increasing role over the long term,” they add.
DeMarchena said that both his personal experience as well as this study will enable him to feel more comfortable performing these procedures going forward. “It’s a very predictable procedure in failed bioprosthetic valves with proper CT planning and echocardiographic and fluoroscopic guidance, and the transeptal technique now is extremely doable,” he commented, adding that for now, however, “we should reserve it for patients that are considered high risk for repeat surgery or that don't have a surgical option.”
It’s too early to say if results like these should affect how surgeons treat patients with mitral valve disease in the first place, DeMarchena noted. “There’s a body of evidence that shows that mitral valve repair in the majority of your patients is a preferred method [over] bioprosthetic valve or mechanical valve replacement in centers that have a great experience in repair, and when you do repairs almost always a ring is involved,” he said. Of note, however, more and more in older patients “surgeons are leaning toward using bioprosthetic valves, partially because of the fact that these valve-in-valves can be performed.”
After a decade of work, “TMVR for failed mitral surgical valves and rings is still ‘under development,’” Webb, Cheung, and Dvir write. “However, these procedures are poised to become the default therapies when mitral implants fail. . . . Hopefully, new tiered systems of care will allow appropriate access to this new therapy while allowing for sufficient expertise to optimize outcomes.”
Photo Credit: Adapted from “Central Illustration.” Yoon S-H, et al. J Am Coll Cardiol. 2017.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Yoon S-H, Whisenant BK, Bleiziffer S, et al. Transcatheter mitral valve replacement for degenerated bioprosthetic valves and failed annuloplasty rings. J Am Coll Cardiol. 2017;70:1121-1131.
Webb JG, Cheung AW, Dvir D. Transcatheter mitral valve replacement when mitral surgery fails: 10 years later. J Am Coll Cardiol. 2017;70:1132-1134.
Disclosures
- Makkar reports receiving grants from Edwards Lifesciences and personal fees from St. Jude Medical and Medtronic.
- Webb and Dvir report serving as consultants for Edwards Lifesciences.
- Cheung reports serving as a consultant for Neovasc.


Comments