Apixaban Curbs Subclinical Thrombosis After TAVI in Patients Without Prior OAC
But prevention of valve thrombosis was not tied to a reduction in events at 1 year, leaving one of TAVI’s key questions unanswered.
Use of apixaban (Eliquis; Bristol-Myers Squibb) following transcatheter aortic valve implantation does not significantly reduce the risk of subclinical valve thrombosis when compared with standard of care, according to a CT substudy from the ATLANTIS trial presented today.
However, the results are nuanced, with investigators reporting an intriguing interaction between thrombus risk and prior oral anticoagulation indication. For patients without an indication for oral anticoagulation, use of apixaban as opposed to antiplatelet therapy was associated with a lower risk of subclinical valve thrombosis as measured by reduced leaflet motion (RLM) and hypoattenuated leaflet thickening (HALT).
By contrast, for those with an indication for oral anticoagulation, apixaban failed to prevent subclinical valve thrombosis when compared with a vitamin K antagonist.
Primary results for ATLANTIS were reported Saturday at the American College of Cardiology 2021 Scientific Session. Lead investigator Gilles Montalescot, MD, PhD (Centre Hospitalier Universitaire Pitié-Salpêtrière, Paris, France), presented the CT substudy, known as ATLANTIS 4D-CT, during the featured clinical research session Saturday.
“This is a study which has two types of patients, and this is the only study where we have patients with an indication for oral anticoagulation and patients without oral anticoagulation,” Montalescot told TCTMD. “What we observed is a reduction in valve thrombosis only in one population, which is a population where the control arm is antiplatelet therapy. So it’s a kind of demonstration that we need anticoagulation—whatever it is—to reduce this risk of valve thrombosis.”
However, whether valve thrombosis is clinically relevant is the million-dollar question. In ATLANTIS 4D-CT, the presence of valve thrombosis 3-6 months after TAVI was not significantly related to clinical events. However, for patients who had grade 3/4 RLM or HALT at 90 days, there were numerically more ischemic events at 1 year (10.7% vs 7.1%), a difference that was not statistically significant.
David Cohen, MD (St. Francis Hospital and Heart Center, Roslyn, NY), one of the discussants, homed in on the clinical relevance of the CT findings, pointing to data from PARTNER 3 and the Evolut Low-Risk Trial showing that “valve thrombosis is something that comes and goes in these patients, unpredictably.” The relationship between valve thrombosis on imaging and clinical outcomes is “not so clear and perhaps weak at best,” he said.
With data from GALILEO, and now from ATLANTIS, Cohen wondered “if it’s time we stop chasing valve thrombosis as an endpoint in transcatheter valve replacement—are we just chasing an imaging finding or is this something still worth trying to prevent?”
Andrew Goldsweig, MD (University of Nebraska Medical Center, Omaha), wondered the same thing. He noted that with the CT substudy, the imaging findings were the primary endpoint while clinical outcomes were a secondary measure. For patients and physicians, what matters most is the clinical outcomes, he stressed.
“There was no difference in the primary endpoint for the entire cohort, but for the patients who were appropriate candidates for antiplatelet therapy, and that was a large cohort, there was less reduced leaflet motion and less HALT with apixaban, but there were no clinical differences,” Goldsweig told TCTMD. “So, the bottom line is that you may change an imaging phenomenon, but you’re not necessarily causing a clinical benefit by using apixaban in this patient population.”
For Montalescot, the jury is still out on whether significant RLM and HALT will ultimately end up being clinical meaningful.
“I think it’s too early to say because we have data out there suggesting there is a link between valve thrombosis and stroke, in registries especially, and we have seen in our study a trend only,” said Montalescot. “We may be underpowered, even if this is the largest study of its kind, nested within a randomized trial, it’s still a limited number of patients, not even 800 patients . . . It’s only 1-year follow-up. What will it look like in 3, 4, or 5 years’ follow-up?”
Nearly 800 Patients With CT Scans
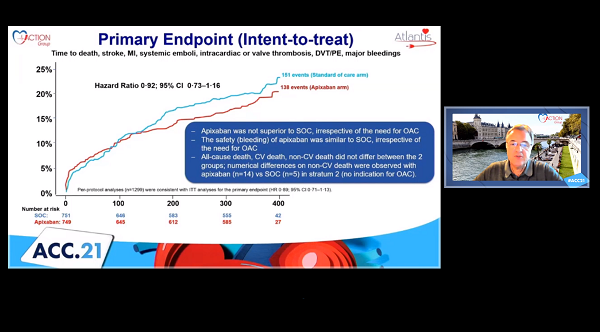
In the main ATLANTIS trial, investigators included 1,510 intermediate- to high-risk patients undergoing TAVI stratified by their need for oral anticoagulation. These patients were then randomized to apixaban 5 mg twice daily or standard care—vitamin K antagonists for those with a clinical indication and antiplatelet therapy for those who did not require anticoagulation. In the overall trial, apixaban was not superior to standard care for the prevention of clinical events, irrespective of the need for oral anticoagulation.
In the substudy, investigators evaluated the incidence of subclinical valve thrombosis at 3-6 months in 762 patients with complete CT scans, including 370 patients randomized to apixaban and 392 randomized to standard care.
For the primary outcome, 8.9% of patients treated with apixaban had moderately restricted/largely immobile leaflets (RLM grade 3/4) or HALT (grade 3/4) compared with 13.0% of patients who received standard care with either antiplatelet therapy or a vitamin K antagonist (OR 0.65; 95% CI 0.41-1.04).
As noted, there was a significant interaction between treatment and the indication for oral anticoagulation (P = 0.0375). In those without an indication for oral anticoagulation, apixaban significantly reduced grade 3/4 RLM or grade 3/4 HALT compared with standard therapy (8.7% vs 15.9%; P = 0.01). In those with an indication for anticoagulation, there was no difference in the incidence of subclinical thrombosis as measured by RLM or HALT between apixaban- and vitamin K antagonist-treated patients.
The prevention of impaired leaflet motion, leaflet thickening, and thrombus “was driven by the effect observed when apixaban was compared with antiplatelet therapy,” said Montalescot. While there was no significant association between valve thrombosis and ischemic clinical events seen here, Montalescot stressed that much is unknown about the phenomenon, including the impact of valve thrombosis on durability.
“This is very important because we have data out there suggesting that thrombosis on the bioprosthetic valves is not good in terms of the durability, that the valves deteriorate faster,” he said, echoing a point raised during yesterday’s presentation by Michael Mack, MD (Baylor Scott & White Heart Hospital, Plano, TX). “It’s only with long-term studies that we can prove that,” Montalescot said.
Joao Cavalcante, MD (Minneapolis Heart Institute, MN), who wasn’t involved in ATLANTIS 4D-CT, agreed there is a struggle to understand the impact of RLM and HALT, but like Mack and Montalescot, said he doesn’t think it should be overlooked. “The formation of thrombus on the bioprosthetic valve is never good,” he said. “I believe that HALT won’t be good long-term for prosthesis durability. . . . I don’t think we can say that this is just an imaging phenomenon. I don’t think we can dismiss it.”
If ATLANTIS 4D-CT had double the number of patients, the study would have likely shown that patients with grade 3/4 RLM or HALT had more ischemic events at 1 year, he said. For Cavalcante, his preference would be to diagnose subclinical thrombosis early and establish a short course of treatment rather diagnose the problem when it’s too late.
At his institute, all TAVI patients are systematically screened for HALT and RLM at 1 month with CT imaging as part of a long-term, prospective valve registry, said Cavalcante. To date, the incidence of HALT is similar to the rate observed in the low-risk TAVI trials, around 20%, and these patients are then treated with at least 3 months of anticoagulation.
“We think it’s a problem that deserves some attention, and we’re tracking these patients long term,” he noted.
Commenting on the analysis for TCTMD, Adnan Chhatriwalla, MD (Saint Luke’s Mid America Heart Institute, Kansas City, MO), said the ATLANTIS substudy reinforces the fact that physicians simply don’t know what to do right now about subclinical leaflet thrombosis. “We want to think it’s a bad thing, but we can’t really seem to pin down the clinical implications of it,” he said. “The nice thing about this study is that there wasn’t a negative impact of being on an anticoagulant like there was in GALILEO, but we’re still not seeing a clinical benefit.”
In his hospital, patients with higher paravalvular gradients 1 month after TAVI will trigger some investigation, leading physicians to “chase down whether they have some restricted leaflet motion or HALT either by echocardiography or CT imaging.” If it’s identified, they will prescribe oral anticoagulation, “but I do wonder whether we’re doing the right thing or not,” said Chhatriwalla. “There are data showing that HALT can come and go with or without anticoagulation.”
While physicians may feel like there should be some clinical implication from RLM and HALT, it’s possible that it goes away on its own and the anticoagulation does nothing, he said. Following the ATLANTIS presentations, Chhatriwalla said his group will likely review their current prescribing patterns to determine if continuing with oral anticoagulation in patients with RLM or HALT on CT is worthwhile.
Track Record of Apixaban Excellent in AF
For Montalescot, apixaban performed relatively well overall in ATLANTIS. There was no safety signal like that seen in GALILEO, so apixaban might be an option in selected patients with an indication for anticoagulation.
“Of course, most of the time, these are A-fib patients, and we know the track record of apixaban in A-fib is excellent,” he said. “Knowing [that] apixaban is much easier to use than warfarin, I think it makes the point for apixaban in this subset of our population.” For those without an indication for oral anticoagulation, despite the reduction in subclinical valve thrombosis, Montalescot urged physicians to be cautious and to wait for more data to emerge about the implications of subclinical valve thrombosis.
To TCTMD, Goldsweig said researchers will be eagerly awaiting the publication of ATLANTIS 4D-CT substudy as they are still grappling with the optimal antithrombotic regimen following TAVI. The POPular TAVI trial recently showed that patients who do not have an indication for oral anticoagulation fare better with aspirin alone than with dual antiplatelet therapy.
“Right now, the take-home is that there might be a difference in imaging with apixaban versus antiplatelet therapy, but antiplatelet therapy is preferable because there’s no difference in antithrombotic outcomes and you’d have to imagine bleeding favors the antiplatelet therapy over the anticoagulant,” he said.
Cohen pointed out that there was a higher rate of noncardiovascular mortality among the apixaban-treated patients without an indication for oral anticoagulation, noting that GALILEO was stopped early because of the mortality signal with rivaroxaban. Montalescot is uncertain what to make of the mortality finding, noting that there was no increased risk of death in the overall population, nor an increased risk of cardiovascular death.
With respect to signal in patients without an indication for anticoagulation, Montalescot said the numbers are small and unrelated to bleeding or ischemic events, such as cancer and sepsis. “Many reasons unrelated to what we have done to these patients,” he said. “It’s very difficult to conclude anything about this signal.”
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Montalescot G, Redheuil A, Vincent F, et al. Apixaban and valve thrombosis after transcatheter aortic valve implantation: the ATLANTIS 4D-CT substudy. Presented at: ACC 2021. May 15, 2021.
Disclosures
- Montalescot reports consulting fees/honoraria from Amgen, AstraZeneca, Bayer, BMS, Boehringer-Ingelheim, Eli-Lilly, MEDTRONIC, Pfizer, and Sanofi-Aventis; and research grants from AstraZeneca, Bayer, BMS, Pfizer, Sanofi-Aventis.




Comments