Aveir DR Dual-Chamber Leadless Pacemaker Looks Solid at 1 Year
The availability of a dual-chamber leadless system is important, but battery longevity remains an issue, one expert says.

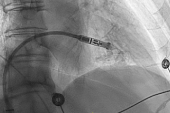
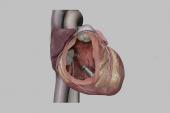
The Aveir DR dual-chamber leadless pacemaker system (Abbott) performs well out to 1 year in patients largely treated for sinus node dysfunction or atrioventricular (AV) block, according to extended follow-up in the Aveir DR i2i Study.
The system, which consists of two devices implanted in the right atrium and the right ventricle, exceeded performance goals for both safety and efficacy in the single-arm, investigational device exemption (IDE) trial at 12 months, consistent with what was seen earlier at 90 days, Reinoud Knops, MD, PhD (Amsterdam UMC, the Netherlands), reported recently at Heart Rhythm 2024 in Boston, MA.
“The safety and performance of a dual-chamber leadless system can be maintained over long periods of time, thus expanding potential indications for leadless pacing,” he concluded.
The Aveir DR i2i Study at 1 Year
Complications from conventional transvenous pacemakers are mostly related to the leads or pockets, and leadless devices are being developed to eliminate these issues, Knops noted. But, he pointed out, single-chamber leadless pacemakers “can’t pace the atrium or deliver reliable AV synchrony at all heart rates, thus limiting indications.”
The Aveir DR is a dual-chamber leadless system that overcomes those hurdles. The two devices that make up the system can communicate wirelessly with each other between the right atrium and the right ventricle—called i2i communication.
The 90-day results from the Aveir DR i2i Study, which included the first 300 de novo implant attempts (mean patient age 69; 38% women), showed a 90.3% system complication-free rate, a 90.2% atrial pacemaker electrical performance success rate, and a 97.3% AV synchrony success rate based on Holter recordings. Both leadless pacemakers were successfully implanted with i2i communication in 98.3% of cases. The findings supported approval of the system by the US Food and Drug Administration in July 2023.
As Knops reported, the 12-month results remained positive. By 1 year, the rate of freedom from system-related complications was 88.6% (95% CI 84.5%-91.8%), with the lower bound of the CI significantly exceeding the performance goal of 76.5% (P < 0.001). There were six complications recorded from day 91 to 365, with four requiring an invasive intervention: two for capture threshold issues and two for symptoms of advanced heart failure. The latter two cases required upgrades to cardiac resynchronization therapy-pacemaker (CRT-P) devices.
The primary efficacy endpoint was the composite success rate of atrial capture and sensing thresholds, which was 92.8% at 1 year (95% CI 89.7%-95.8%). The lower bound of the CI significantly exceeded the performance goal of 80.0% (P < 0.001). There were only three revisions for high thresholds or noncapture.
Through 1 year, the success of communication between implants was around 90%, “which is a very good result in my opinion,” Knops said.
What About Battery Longevity?
Knops acknowledged that all of that communication between the two implants uses up a lot of battery power, with a mean projected battery life of 6.4 years for the atrial device and 11.3 years for the ventricular device. If the system can be programmed to single-chamber mode at least once, however, those figures rise to 9.8 and 19.1 years, respectively.
During a panel discussion after his presentations, Knops indicated that lessening the degree of communication might be an option for some patients. “If you do that, you do save a lot of energy,” he said. “And certainly, the patients that can function perfectly with other pacing modes, those are ideal candidates to stop the communication and save a lot of energy, and then you see that the longevity of the device outperforms what we currently know of other leadless pacemakers.”
It’s hard to justify a dual-chamber leadless system for everyone because we’re going to end up removing a lot of those atrial devices and replacing them. Mikhael El-Chami
Commenting for TCTMD, Mikhael El-Chami, MD (Emory University, Atlanta, GA), said battery longevity is a key issue, noting that use of dual-chamber versus single-chamber programming reduced the life of the batteries by about 40%. That’s important, he said, because after 5 or 6 years, clinicians will have to decide what to do when the atrial battery is depleted.
When thinking about maximizing battery longevity and minimizing complications, the best approach likely depends on the specifics of each situation, El-Chami indicated. For a patient with sinus node dysfunction and an intact conduction system, for instance, it might be appropriate to implant only the atrial device and then add the ventricular device if the patient eventually develops conduction system disease. On the other hand, for a patient with intermittent pauses, it might make more sense to implant a leadless pacemaker only in the ventricle and program it for demand pacing. And then some patients will require the dual-chamber system with a shorter battery life.
“But I think given the impact on the battery longevity, in my mind it’s hard to justify a dual-chamber leadless system for everyone because we’re going to end up removing a lot of those atrial devices and replacing them,” El-Chami said. For some patients, such as those on dialysis or those with a history of infections, “you might take the drawback of a shorter battery life and use that system because it has [a] lower incidence of infection compared to a transvenous system.”
Also working against wider adoption of the dual-chamber leadless system at this point is the “prohibitive cost,” El-Chami said.
Still, he said, it’s important to remember that current leadless pacing systems are early in their development and as the technology matures, there will be improvements. El-Chami said he will be watching to see if battery longevity can be improved or if devices can be made with rechargeable batteries, which will eliminate these concerns. In addition, there are questions around whether leadless systems can be developed to perform CRT or to be used for conduction-system pacing.
For now, “the availability of a dual-chamber leadless system is an important step forward with this technology,” El-Chami said. “It’s important that it is available. I do not believe at this point it will take over the traditional transvenous system but it’s definitely good to have it as part of the armamentarium of pacing technology because some patients definitely will benefit from a leadless system.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Knops R. One-year safety and performance outcomes from a clinical study of a dual-chamber leadless pacemaker system. Presented at: HRS 2024. May 18, 2024. Boston, MA.
Disclosures
- The Aveir DR i2i IDE study was funded by Abbott.
- Knops reports receiving stock options from AtaCor Medica and honoraria or speaking/consulting fees from Abbott, Boston Scientific, Cairdac, Kestra, and Medtronic.



Comments