Cardioband Reduces Severe Tricuspid Regurgitation at 6 Months, With Good Safety: TRI-REPAIR
The time is now for head-to-head comparison studies of available tricuspid valve reconstruction devices, according to one expert.
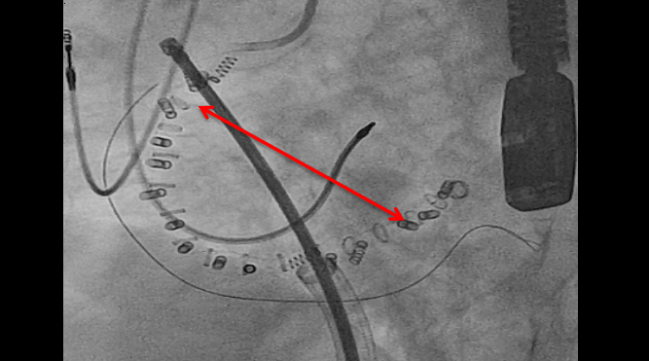
Six-month results from the TRI-REPAIR study show potential for the Cardioband percutaneous annuloplasty ring (Edwards Lifesciences) to reduce moderate to severe functional tricuspid regurgitation (TR) among symptomatic patients.
Findings from the TRI-REPAIR study were published online this week in Open Heart and initially presented at PCR London Valves in September. Lead researcher Georg Nickenig, MD (University Hospital Bonn, Germany), and colleagues say that “further studies are warranted to validate these initial promising results.”
While the device received CE Mark approval in Europe for use in the tricuspid valve last year and has been approved there to treat mitral regurgitation using a slightly different delivery system since 2015, it is not currently commercially available in the United States.
“The TRI-REPAIR study is an important addition to current knowledge,” writes Jeffrey Borer, MD (SUNY Downstate Medical Center, Brooklyn, NY), in an accompanying editorial. “There really is not a perfect tricuspid valve repair method, particularly when we're talking about catheter-based repair,” he specified to TCTMD. “This is another one. So this one may turn out to be as good as or better than the previously existing approaches, and that makes it important.”
Six-month Findings
The percutaneously delivered device is an incomplete, semirigid annuloplasty ring with a gap for the atrioventricular (AV) node. It uses a series of anchors to affix to the tricuspid valve annulus. TRI-REPAIR was conducted between October 2016 and July 2017 at four European centers, where researchers enrolled 30 patients with severe functional TR, of whom 83% had NYHA class III/IV heart failure.
Technical success, defined as successful access, device deployment, and positioning, was achieved in all patients. Two patients died within 30 days and one death was deemed device-related, but there were no instances of MI or device-related cardiac surgeries. Three patients reported major serious adverse events (13.3%), and other periprocedural adverse events deemed clinically important included four bleeding complications, three right coronary artery complications, two ventricular arrhythmias, and one instance of AV block.
By 6 months, mean annular diameter remained significantly reduced compared with baseline (41.6 vs 37.8 mm; P = 0.0014). Tricuspid regurgitation also decreased as per a wide variety of echocardiographic parameters, and only five patients (28%) presented with severe or greater tricuspid regurgitation at 6 months.
“Circumferential annular reduction with the use of multiple anchors may allow for more significant TR reduction compared with other transcatheter annular repair approaches,” write Nickenig and colleagues. “The sustained reduction of these parameters with the transcatheter tricuspid system at 6 months are encouraging, and additional studies are warranted.”
At 6-month follow-up, 15 (60%) and 7 (28%) of patients were deemed NYHA functional class II and I, respectively (P < 0.0001). Additionally, both mean 6-minute walk distance and KCCQ quality-of-life scores improved significantly from baseline.
Time for Head-to-Head Comparisons
In addition to studies of this device’s safety and effectiveness, the researchers write that much remains to be discovered about how tricuspid regurgitation actually effects morbidity and mortality.
“Our current knowledge has not unequivocally proven whether TR directly drives poor outcomes or whether TR is only a surrogate of other concomitant disorders such as right- or left-heart failure. This uncertainty and the putative tremendous unmet need increase the urgency to effectively and safely treat TR via novel therapies,” they stress.
The potential benefits of the Cardioband over other options, the authors say, are that “it effectively addresses the leading pathophysiology of functional TR, which is annular dilatation. It does not require large vascular or even transatrial access, and it allows for future additional treatment modalities. This may be an especially attractive option in patients with extensive coaptation defects where annular reduction with the system could be combined with leaflet therapy in order to completely abolish TR.”
Photo Credit: von Bardeleben RS. Transcatheter TR Solution 5: Cardioband. Presented at: TCT 2017. November 1, 2017. Denver, CO.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Nickenig G, Weber M, Schueler R, et al. 6-month outcomes of tricuspid valve reconstruction for patients with severe tricuspid regurgitation. J Am Coll Cardiol. 2019;73:1905-15.
Borer JS. Application of transcatheter repair to tricuspid regurgitation. J Am Coll Cardiol. 2019;73:1916-18.
Disclosures
- Nickenig reports receiving research funding from the Deutsche Forschungsgemeinschaft (DFG), the Federal Ministry of Education and Research (BMBF), The European Union, Abbott, AGA Medical, AstraZeneca, Bayer, Berlin Chemie, Biosensus, Biotronic, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi-Sankyo, Edwards Lifesciences, Medtronic, Novartis, Pfizer, Sanofi, and St. Jude Medical; receiving honoraria for lectures or advisory boards from Abbott, AGA Medical, AstraZeneca, Bayer, Berlin, Cardiovalve, Berlin Chemie, Biosensus, Biotronic, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi-Sankyo, Edwards Lifesciences Medtronic, Novartis, Pfizer, Sanofi, and St. Jude Medical; and participating in clinical trials for Abbott, AGA Medical, AstraZeneca, Bayer, Berlin Chemie, Biosensus, Biotronic, Bristol-Myers Squibb, Boehringer Ingelheim, Cardiovalve, Daiichi-Sankyo, Edwards Lifesciences, Medtronic, Novartis, Pfizer, Sanofi, and St. Jude Medical.
- Borer reports serving as a consultant for Takeda, Novartis, AstraZeneca, BioMarin, GlaxoSmithKline, Servier, ECG, and CellAegis.


Comments