CHOICE-2 Bolsters Intra-arterial Lytics After Stroke Thrombectomy
With the study adding to a mix of conflicting trial results, it’s not yet time to update guidelines, Shyam Prabhakaran says.
NEW ORLEANS, LA—Administering intra-arterial alteplase after successful endovascular thrombectomy in patients with acute ischemic stroke improves functional outcomes and reduces persistent hypoperfusion, according to the results of the CHOICE-2 trial.
That was achieved without increasing the risk of symptomatic intracranial hemorrhage (sICH), Ángel Chamorro, MD, PhD (Hospital Clinic of Barcelona, Spain), reported here at the International Stroke Conference. All-cause mortality was higher when intra-arterial therapy was added, although the difference may have been related to a lower-than-expected rate in the control group rather than an increase with the intervention.
The trial investigators “believe that CHOICE-2 supports the administration of intra-arterial alteplase after successful thrombectomy in selected patients,” Chamorro said.
Several prior trials have explored the impact of intra-arterial thrombolytics after successful thrombectomy, with mixed results. The first CHOICE trial made headlines when it showed an absolute 18% increase in the proportion of patients with an excellent functional outcome with use of intra-arterial alteplase. The findings were not, however, considered definitive because the study was stopped prematurely. Over the next several years, three trials—POST-UK and POST-TNK, along with ATTENTION-IA—failed to find a benefit with intra-arterial lytics, while another two, ANGEL-TNK and PEARL, yielded positive results.
In the latest guideline on the early management of acute ischemic stroke, released last week by the American Heart Association/American Stroke Association, the conflicting evidence has been distilled into a class 2b recommendation stating that it may be reasonable to use adjunctive intra-arterial thrombolytics to improve cerebral perfusion and 90-day functional outcomes in patients who undergo thrombectomy that results in complete or near-complete reperfusion.
Shyam Prabhakaran, MD (University of Chicago Medicine, IL), chair of the guideline writing group, told TCTMD the magnitude of the improvement in functional outcome seen in CHOICE-2 “opens the door” to this adjunctive treatment approach. “We know that small clots in the distal vasculature are very hard to approach mechanically and that lytic agents could get into that microcirculation and dissolve them, Prabhakaran commented.
That said, he cautioned against interpreting CHOICE-2 as guideline-changing, pointing to the other conflicting trial results.
“I think maybe the debate will rage on a bit more, and this gives people more ammunition to do this type of study on a larger scale. Probably an international study is needed,” he said.
The CHOICE-2 Trial
Even with a successful angiographic result, fewer than one-third of patients who undergo stroke thrombectomy achieve an excellent functional outcome, defined as a modified Rankin Scale score of 0 to 1, Chamorro noted, adding that the main hypothesis to explain that phenomenon is that microvascular hypoperfusion persists after opening up the vessel.
“Back in 2020, we were the first to propose that the outcome of a patient with a stroke could improve beyond angiographic success [following thrombectomy] with the administration of intra-arterial alteplase,” Chamorro said.
Because the results of CHOICE were considered preliminary, the investigators designed CHOICE-2 to validate the findings in a larger phase III population. The trial, conducted at 14 stroke centers in Spain, included adults with acute ischemic stroke caused by a large-vessel occlusion who were treated with thrombectomy within 24 hours of onset and achieved successful reperfusion (eTICI 2b-3).
After confirmation of successful reperfusion, 440 patients were randomized to intra-arterial alteplase 0.225 mg/kg (maximum dose 20 mg) given in a 15-minute infusion or no intra-arterial lytics. The primary analysis included 436 patients (median age about 76 years; 51% women) who adhered to the protocol. Median NIHSS score at baseline was 15.
Nearly two-thirds of patients (63%) received IV thrombolysis before thrombectomy. More than half of patients (57%) achieved complete reperfusion after the procedure (eTICI 3), with another 20% achieving 90% to 99% reperfusion (eTICI 2c).
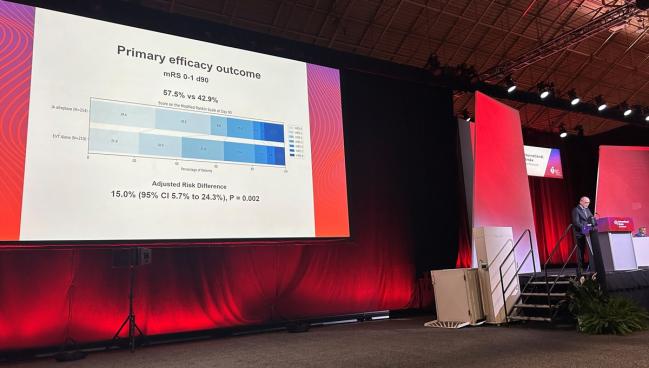
An excellent functional outcome at 90 days, the primary endpoint, was observed in 57.5% of patients who received adjunctive intra-arterial alteplase and 42.9% of those who underwent thrombectomy alone, with an adjusted risk difference of 15.0%. That equates to a number need to treat of 7. The findings were consistent across subgroups.
CT perfusion imaging performed at 36 hours showed that patients who received intra-arterial treatment were less likely to have persistent hypoperfusion (29% vs 51%; P < 0.001).
Among secondary outcomes, adjunctive therapy was associated with significantly better self-reported quality of life based on the EQ-5D-5L assessment (P = 0.02) and, among survivors, an increased likelihood of having a Barthel index of 95 to 100 at 90 days (72% vs 62%; P = 0.04).
There was no difference between the intervention and control arms in sICH (1.4% vs 0.5%; P = 0.33). The rate of all-cause death was “very low” in the alteplase arm and “extremely low” in the control arm, Chamorro said, leading to a significantly higher rate among those who receive intra-arterial lytics (12.1% vs 6.4%; P = 0.04).
The mortality finding “isn’t directionally related to the symptomatic hemorrhage rates, because they were equivalent,” Prabhakaran said. “It’s hard to understand what could have been causing the deaths if it’s not hemorrhage. So it is puzzling, but it also makes you want to see more of these studies done. Because when you have mixed signals like that, you definitely want to make sure that you’re not overenthusiastic after one study.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Chamorro Á. CHOICE-2 trial: a randomized trial of intraarterial alteplase after successful thrombectomy. Presented at: ISC 2026. February 4, 2026. New Orleans, LA.
Disclosures
- CHOICE-2 was sponsored by Fundació de Recerca Clínic Barcelona-IDIBAPS and funded by the Spanish Ministry of Health.
- Chamorro and Prabhakaran report no relevant conflicts of interest.




Comments