CHOICE: Intra-arterial tPA Enhances Benefit of Stroke Thrombectomy
Physicians will start thinking more about giving adjunctive tPA unless there’s a clear contraindication, one expert predicts.
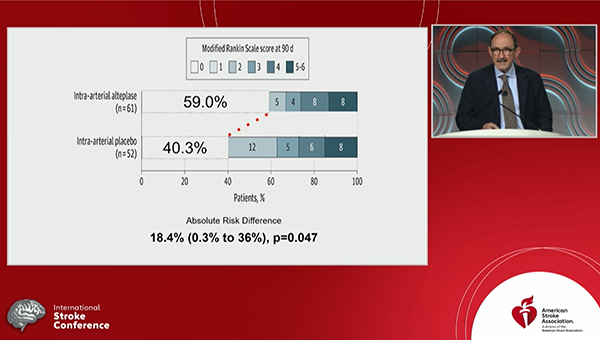
Administering intra-arterial tissue plasminogen activator (tPA; alteplase) after successful thrombectomy boosts outcomes in patients with acute ischemic strokes caused by large-vessel occlusions, the randomized CHOICE trial shows.
Those who received intra-arterial tPA were significantly more likely to have an excellent neurological outcome—defined as a modified Rankin Scale (mRS) score of 0 to 1—at 90 days compared with those who received placebo (59.0% vs 40.4%; P = 0.047), Ángel Chamorro, MD, PhD (Hospital Clinic of Barcelona, Spain), reported Thursday at the International Stroke Conference (ISC) 2022.
That benefit was not explained by angiographic improvements, Chamorro noted. “The clinical versus angiographic mismatch encountered in the study stresses the limitations of conventional angiography to monitor these patients and highlights the relevance of the microcirculation to improve the clinical efficacy of mechanical thrombectomy.”
However, he advised caution in interpreting the results, published simultaneously online in JAMA, because the trial was stopped early, and he made a pitch for a CHOICE 2 trial to validate the findings.
But Louise McCullough, MD, PhD (University of Texas Health Science Center at Houston), chair of ISC 2022, called CHOICE an impactful study that demonstrates the benefit of using intra-arterial tPA “almost like a cleanup” to address pieces of clot that remain behind after thrombectomy. “You can get the main clot out but some may fragment and go distally and may cause no reperfusion in the more peripheral vessels,” leading to the no-reflow phenomenon, McCullough explained. “That’s become a big issue in acute stroke care: how do we get complete recanalization, not just TICI 3, but how do we get those smaller vessels open?”
For that reason, she predicted that CHOICE will influence practice. “People are going to start thinking about giving a little adjunctive tPA after thrombectomy unless there’s a clear contraindication,” she told TCTMD, noting that the addition of intra-arterial tPA didn’t increase bleeding.
The CHOICE Trial
Mechanical thrombectomy results in successful reperfusion in 71% of patients, but only about 27% of patients who undergo the procedure are disability-free (mRS 0 to 1) at 3 months, Chamorro said. Undoubtedly, some of the patients with poorer outcomes had irreversible brain damage at the time of clot removal. It’s also possible, though, that incomplete microcirculatory reperfusion—despite complete recanalization on angiography—is contributing.
The investigators thought this could be a frequent and clinically meaningful problem, and one that could be treatable by administering intra-arterial tPA after thrombectomy to allow the drug easier access to the distal microcirculation.
To test this idea, they designed CHOICE, a phase IIb trial conducted at seven stroke centers in Catalonia, Spain. Patients who underwent thrombectomy resulting in successful reperfusion (mTICI 2b/3) were randomized to receive intra-arterial alteplase or placebo after the procedure. Alteplase was infused at a dose of 0.225 mg/kg (maximum 22.5 mg) over 15 or 30 minutes.
The trial was stopped early after only 60% of the planned patients were enrolled due to problems with placebo availability and study enrollment related to the COVID-19 pandemic. Ultimately, 121 patients (mean age 71 years; 47% women) entered the trial, and 113 were treated as randomized. The median NIHSS score was 14, and the median ASPECTS was 9. Most patients (57%) received IV alteplase before thrombectomy.
The proportion of patients with an excellent neurological outcome at 90 days was an absolute 18.4% higher after the addition of intra-arterial tPA, although the confidence interval around that figure was wide (0.3% to 36.0%).
There were no differences between trial arms in various angiographic measures, including improvement in the expanded TICI score, the infarct expansion ratio, the proportion of patients with infarct expansion, or infarction volume at 48 hours. The intervention was shown to be safe, with no symptomatic intracranial hemorrhages in the alteplase group and two in the placebo group. The 90-day mortality rate was nonsignificantly lower in the tPA arm (8% vs 15%).
In an accompanying editorial, Pooja Khatri, MD (University of Cincinnati, OH), says “it is provocative to consider whether, as the authors suggest, the improved outcomes observed may have been related to an amelioration in the microcirculatory reperfusion.”
She indicates, however, that additional research is needed to confirm that idea. “To exclude the possibility that the benefit of alteplase is in its effect on the remaining visualized clot, the concept would need to be tested in a larger cohort of patients with complete angiographic reperfusion,” defined as an expanded TICI score of 3, Khatri says, noting that CHOICE included only 26 patients with complete angiographic reperfusion.
Overall, the trial “suggests that additional reperfusion therapy may be warranted after relatively successful mechanical thrombectomy of large-vessel occlusions, whether to treat the residual primary thrombus, more distal arterial occlusions, or perhaps even microthromboses,” Khatri writes.
“This approach runs counter to the recent movement to consider bypass of intravenous alteplase altogether in thrombectomy-eligible patients, and suggests that additional or perhaps more-targeted thrombolysis will be the most beneficial approach,” she continues. “Further studies testing current thrombolytic agents, novel clot-dissolving agents, and other adjunctive antithrombotic and anti-inflammatory agents are needed.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Renú A, Millán M, San Román L, et al. Effect of intra-arterial alteplase vs placebo following successful thrombectomy on functional outcomes in patients with large vessel occlusion acute ischemic stroke: the CHOICE randomized clinical trial. JAMA. 2022;Epub ahead of print.
Khatri P. Intra-arterial thrombolysis to target occlusions in distal arteries and the microcirculation. JAMA. 2022;Epub ahead of print.
Disclosures
- The study was supported by Fundació La Marató de TV3 and by the Spanish Ministry of Health cofinanced by the European Regional Development Fund. The study medication and placebo were provided by Boehringer Ingelheim. This work was partially developed at the Centre de Recerca Biomèdica Cellex, Centre Esther Koplowitz, IDIBAPS Barcelona, CERCA Programme/Generalitat de Catalunya.
- Chamorro reports a patent pending (Ox-02:2021/14997) with FreeOx Biotech.
- Khatri reports a grant from Cerenovus; personal fees from Bayer, Lumosa, Basking Biosciences, Diamedica, and UpToDate; and funds to her department from Genentech (lead principal investigator of the PRISMS trial).




Comments