Complete 2-Year Data From Evolut Low-Risk Trial ‘Reassuring’ for TAVI
The findings confirm primary outcomes seen in a preliminary Bayesian analysis, but also the higher pacemaker and PVL rates.
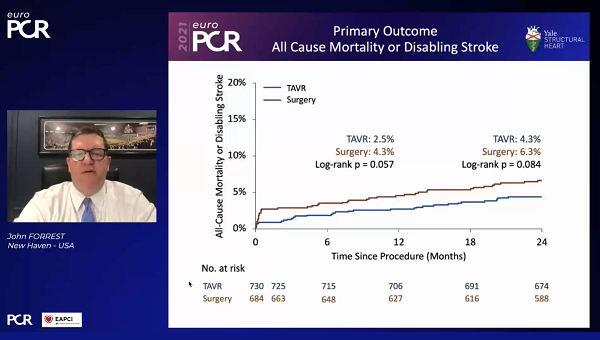
(UPDATED) Among patients at low risk for surgery, TAVI with the Evolut prosthetic valve (Medtronic) is noninferior to surgery with regard to the endpoint of death or disabling stroke at 2 years, according to additional data from the Evolut Low-Risk Trial.
The findings strengthen the original results, presented at American College of Cardiology (ACC) 2019 meeting and simultaneously published in the New England Journal of Medicine, which showed noninferiority of TAVI compared with surgery for death and disabling stroke at 24 months in a Bayesian analysis once 850 of the 1,414 enrolled patients had reached 12-month follow-up.
In the complete analysis, presented by John Forrest, MD (Yale School of Medicine, New Haven, CT), yesterday at EuroPCR 2021, all patients had completed 2-year follow-up, representing 97.3% and 92.3% of the patients originally randomized to TAVI and surgery, respectively. Longer-term follow-up will evaluate patient endpoints out to 10 years, said Forrest.
“We can be reassured that these patients are doing very well and I think as we move to younger ages, the longer-term outcomes become critically important,” Forrest said in a discussion of the results. “I also think that we need to be realistic here. We’re really going to be interested in 5- and 10-year outcomes and potentially even thereafter. What happens to these valves when they eventually fail? Are superior hemodynamics going to give us longer valve durability in some way or are there going to be other unforeseen things that come up 10 years out? We don’t yet know those answers.”
“It was very reassuring to confirm that the real, observed outcomes reported in this study were in line with the expected outcomes reported after estimation by Bayesian methods, with even lower rates of events than expected,” agreed Nicolas Dumonteil, MD (Clinique Pasteur, Toulouse, France), who commented on the study for TCTMD via email. “This Bayesian method is a complex statistical method that all physicians are not able to critically review, so I think it was fundamental to report the actual outcomes.”
Bernard Prendergast, MD (St Thomas' Hospital, London, England), and Christopher Cook, MD (Essex Cardiothoracic Centre, England), who discussed the results following the virtual presentation, suggested that these full results serve to authenticate the reporting strategy adopted by the investigators.
The Bayesian statistical analysis “did generate some criticism and discussion whether this was an appropriate methodology compared with traditional Kaplan-Meier analyses,” Prendergast noted. “Indeed, some people accused the investigators of gaming it with this form of statistical analysis.” The results presented today would suggest otherwise.
Cook, likewise, called it “a fascinating opportunity to assess how well the predicted clinical outcomes compared with the actual clinical outcomes,” adding that the Evolut trial did just that. “This validates the use of the original Bayesian methods to estimate TAVI outcomes in low-risk TAVI patients, and indeed it may act as an example of where Bayesian methods can be safely applied in order to fast track potentially transformative procedures and technologies to our patients,” he said.
At 2 years, the primary outcome of all-cause mortality and disabling stroke was reported in 4.3% of TAVI and 6.3% of surgically-treated patients at 2 years, a nonstatistically significant difference, Forrest reported, noting that the curves “remained almost parallel” from the 1-year mark onward. Constraints for noninferiority but not superiority were met, he said. Also, a landmark analysis conducted between 1 and 2 years showed no difference between the study groups after the 1-year mark (1.9% vs 2.1%; P = 0.742).
Breaking down the individual endpoints, both all-cause mortality (3.5% vs 4.4%; P = 0.366) and disabling stroke (1.5% vs 2.7%; P = 0.119) were comparable between the study groups at 2 years.
The need for permanent pacemaker, however, was significantly higher in the TAVI arm (21.1% vs 7.9%). “I do certainly think we’ve made improvements since the study was completed about pacemakers, understanding it better, in particular using the [right anterior oblique] cusp overlap has been shown to significantly reduce the incidence of pacemaker with the Evolut valve,” Forrest commented. “And I think we’ve seen the pacemaker rates drop over the 2 years since the study was completed.”
Also consistent with prior studies, the incidence of hospitalization for heart failure was numerically lower in the TAVI arm (5.3% vs 7.1%). TAVI was superior to surgery in terms of valve hemodynamics, with mean gradients remaining in the single digits for TAVI and valve areas averaging 2.2 cm2 (P < 0.001 for all time points).
The findings will “reinforce the scientific rationale physicians use in their everyday clinical practice, within their heart teams, to offer TAVR to low-risk patients having now at short- and mid-term a quite clear overview of benefits and risks as compared to SAVR,” Dumonteil said. “It allows us more and more in this low-risk stratum of patients, for which surgery is still a good option, to tailor the proposed therapy.”
“It’s important to note that there are differences in these patients and we don't know yet what the long-term outcomes will be for differences that we see in hemodynamics, durability, the incidence of pacemaker, patient-prosthesis mismatch, paravalvular leak, or other things,” Forrest said in a media briefing: hence the planned 10-year follow-up.
He also highlighted that patients with bicuspid anatomy, or anatomy otherwise unsuitable for TAVI, were excluded from the trial. This underscores “the importance of a heart team workup for these patients,” Forrest said. “But with appropriately suited patients, I think we have really nice results at 2 years and we'll be following them to see what the impacts of some these differences are.”
In addition to learning about how these valves may deteriorate over time, Dumonteil said “we have to understand that these very positive outcomes were obtained without the use of latest-generation platforms or implantation technique (cusp overlap implantation technique to reduce pacemaker rate, per example). So one could expect even better outcomes after TAVR with those current devices/techniques.”
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Forrest J. Complete 2-year follow-up from the Evolut low risk trial. Presented at: EuroPCR 2021. May 18, 2021.
Disclosures
- The study was funded by Medtronic.
- Forrest reports receiving grant support, serving on the advisory board, and proctoring for Edwards Lifesciences and Medtronic.
- Dumonteil reports receiving consultancy a d proctoring fees from Abbott Vascular, Boston Scientific, Edwards Lifesciences, and Medtronic related to TAVI.




Comments