Coronary Sinus Reducer ‘Promising’ for Refractory Angina: Meta-analysis
Effects are more modest in placebo-controlled versus nonrandomized studies, with experts calling for further research.
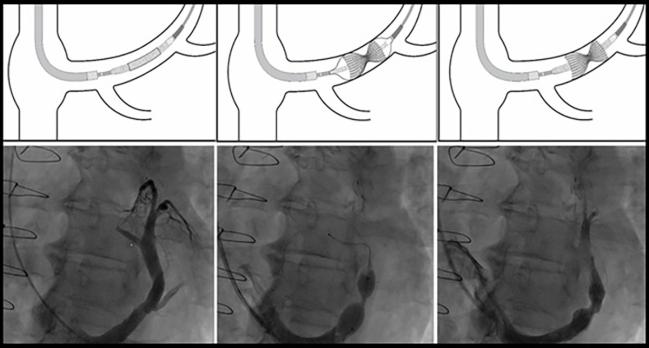
Photo Credit: Adapted from Verheye S. The coronary sinus reducer. Presented at: TCT 2023. San Francisco, CA.
An implantable coronary sinus reducer (CSR)—Shockwave Reducer (Shockwave Medical)—likely eases symptoms in patients with refractory angina, but the impact is smaller in sham-controlled trials than is suggested in nonrandomized studies, according to a meta-analysis.
With the CSR, 26% of patients saw an improvement of at least one Canadian Cardiovascular Society (CCS) angina class and 17% had an improvement of at least two classes in placebo-controlled analyses. Both figures represent roughly one-third of the effect observed in single-arm studies, researchers led by Utkarsh Ojha, MBBS (Queen Elizabeth The Queen Mother Hospital, Margate, England), and Muhammad Mohsin, BSc (Imperial College London, England), report.
The findings, which were published in the August 11, 2025, issue of JACC: Cardiovascular Interventions, show that “CSR implantation is safe and feasible and demonstrates promising antianginal efficacy in patients with refractory angina,” the authors write. “However, uncertainty in current efficacy findings and susceptibility of effectiveness data to nonphysical influences preclude definitive conclusions about clinical utility, warranting larger placebo-controlled trials.”
Despite acceptable safety, they say, there are still some risks of the procedure that “necessitate clear benefit justification” before broader clinical use. The Shockwave (formerly Neovasc) Reducer is the only commercially available CSR, although others are in development. It is on the market in Europe and elsewhere, but it remains investigational in the United States and Canada.
More data on the device will be forthcoming from the ongoing sham-controlled COSIRA-II trial, set to enroll an estimated 380 patients with severe angina despite optimal medical therapy and epicardial disease with no revascularization options across the US.
But in the meantime, “there is definitely more and more evidence that in patients who have no other options for angina relief, this is a device that can be used, and we’ve certainly been using it in the UK,” senior author Rasha Al-Lamee, MBBS, PhD (Imperial College London, England), who headed the ORBITA-COSMIC trial of the Shockwave Reducer (Shockwave Medical), told TCTMD. Al-Lamee said she expects the procedure to become even safer with future iterations or types of CSR.
Commenting for TCTMD, Jaikirshan Khatri, MD (NewYork-Presbyterian/Weill Cornell Medicine, New York, NY), said the meta-analysis “is supportive of what we already understand about the device—that there clearly is some clinical response. What we still need to understand is: who is most likely to respond and how can we select patients that are going to have the most benefit from the procedure?”
Effectiveness vs Efficacy
There are limited therapeutic options for patients with refractory angina despite maximal antianginal treatment, and the CSR has emerged as an option for symptom relief. “This hourglass-shaped, stainless-steel mesh is percutaneously implanted to create a controlled narrowing in the coronary sinus, hypothesized to redistribute blood flow from well-perfused areas to ischemic myocardial regions,” the investigators say.
Previous meta-analyses of the technology have focused on single-arm studies, with the results reflecting effectiveness, combining not only the effect of the device, but also variables like bias and confounding. An assessment of true treatment efficacy, on the other hand, requires RCTs.
In this new meta-analysis, “we wanted to understand the difference between the unblinded and the blinded effect sizes, with the knowledge that blinded effect sizes are usually smaller,” Al-Lamee said.
The analysis included three double-blind, placebo-controlled RCTs—COSIRA, CROSSROAD, and ORBITA-COSMIC—with a total of 180 patients plus 13 single-arm studies with a total of 668 patients. Mean follow-up was 7.2 months.
The unweighted implantation success rate was 98.3%, with no periprocedural mortality or stroke. The most common complication was CSR migration (1.5%), which was defined as dislocation of the device or embolization. Overall safety was favorable, but the procedure “carries nonnegligible risks, including cardiac tamponade and device embolization, underscoring the need for robust efficacy evidence,” the researchers say.
On that front, there were significant improvements across some—but not all—outcomes in both single-arm studies and RCTs. CCS angina class improved, with smaller gains in the randomized trials.
On the Seattle Angina Questionnaire, all domains showed improvement with CSR implantation in single-arm studies, but there were no significant changes in physical limitation, angina stability, angina frequency, treatment satisfaction, or quality of life in placebo-controlled analyses. “However, individual RCTs provide statistical evidence of benefit in quality of life and angina frequency, with upper uncertainty limits exceeding this threshold, preserving the possibility of meaningful efficacy in these domains,” the authors write.
Treadmill exercise time increased after CSR implantation, with a placebo-controlled pooled estimated gain of 49.62 seconds (P = 0.04). But confidence intervals around the estimate were wide and the researchers note that larger trials are necessary because “our meta-analysis supports a benefit but cannot precisely quantify its magnitude.”
In terms of measures of myocardial ischemia, there was no change in placebo-controlled echocardiographic wall motion score index and “insufficient evidence of improvement” for global perfusion reserve assessments.
“What we were trying to understand was where we can expect to see the effect of the device, and it seems to be mainly working in terms of symptom relief, while the magnitude of improvement in exercise time was harder to quantify with substantial uncertainty around the estimate of effect,” Al-Lamee said.
Open Questions
One of the unresolved issues with the CSR is its mechanism of action. “This bit has been one of the interesting parts of this device because we are not used to angina relief coming from a device that sits within the venous circulation. Many people have felt that understanding exactly how this device works is not intuitive,” Al-Lamee said.
Another major question, Khatri added, is whether the CSR would be more suited to patients with microvascular dysfunction. “It could be that really what we’re doing is we’re treating patients with epicardial disease and the responders are people that have concomitant microvascular disease,” he said. “We don’t really know what we’re doing. Are we treating patients with epicardial ischemia? Are we treating people with microvascular dysfunction? Are we treating both? Is the response in somebody who has an overlap of both of these entities?”
There is ongoing research to address this question. The SERRA-I early feasibility study, for instance, will test the novel A-FLUX self-expanding CSR (VahatiCor) in patients with isolated microvascular disease. “That’ll be very interesting because that also potentially could lead to some treatment options for patients that to date we don’t have any other medical therapy for,” Khatri said.
Looking Forward
Khatri predicted that the CSR “absolutely is going to have a role in clinical practice” in the future, with COSIRA-II and SERRA-I hopefully helping to fill in some of the knowledge gaps around the mechanism of action and patient selection.
The device hasn’t changed much in more than a decade, so “there’s also opportunities to make an easier to use device that might be more forgiving for deployment and could hopefully make the procedure safer,” he said. “I think that that could potentially sway the equation in favor of treating patients rather than being more conservative over concerns about complications.”
Overall, Khatri said, “I think that patients with refractory angina are naturally going to gravitate towards this therapy because they’ve run out of options.
“But for a microvascular disease patient who doesn’t have epicardial disease, this also is an attractive treatment option where right now there are no procedural treatments available,” he continued. “There’s a limited role for medical therapy and no other options beyond that. And if we can come up with a device that’s efficacious as well as safe, I think that that could be very attractive for that subset of patients as well.”
In an accompanying editorial, Michael Vavuranakis, MD, PhD, and Deepak Bhatt, MD (both from Icahn School of Medicine at Mount Sinai, New York, NY), say “there may be a role for the CSR in future clinical practice, particularly for patients with refractory angina, as it might provide improvements in symptomatology and quality of life.”
Several issues need to be addressed, they say, pointing to the need for criteria for patient selection and procedural success, a definition of optimal antithrombotic therapy after implantation, and data on potential de-escalation of antianginal medications when symptoms resolve.
“Healthcare systems should resist the pressure to approve and reimburse devices such as the CSR before robust data supporting utilization,” Vavuranakis and Bhatt write.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Ojha U, Mohsin M, Macierzanka K, et al. Safety, efficacy, and effectiveness of coronary sinus reducer implantation in refractory angina: a meta-analysis. JACC Cardiovasc Interv. 2025;18:1864-1877.
Vavuranakis M, Bhatt DL. Relieving the pressure: evaluating the coronary sinus reducer for refractory angina. JACC Cardiovasc Interv. 2025;18:1878-1880.
Disclosures
- Al-Lamee reports having served on advisory boards for Janssen Pharmaceuticals, Abbott, and Philips; having received speaker fees from Abbott, Philips, Medtronic, Servier, Omniprex, and Menarini; and having received consulting and speaker fees from Shockwave Medical (outside the submitted work).
- Bhatt reports having been on advisory boards for Angiowave, Bayer, Boehringer Ingelheim, CellProthera, Cereno Scientific, E-Star Biotech, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, NirvaMed, Novo Nordisk, Stasys, and Tourmaline Bio; having been on the board of directors of the American Heart Association New York City, Angiowave (stock options), Bristol Myers Squibb (stock), DRS.LINQ (stock options), and High Enroll (stock); having received consulting fees from Broadview Ventures, Corcept Therapeutics, GlaxoSmithKline, Hims, SFJ, Summa Therapeutics, and Youngene; having been on data monitoring committees with Acesion Pharma, Assistance Publique Hôpitaux de Paris, the Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic, Contego Medical (Chair, PERFORMANCE 2), the Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical; for ALLAY-HF, funded by Alleviant Medical), Novartis, the Population Health Research Institute, and Rutgers University (for the NIH-funded MINT trial); having received honoraria from the American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), the Arnold and Porter law firm (work related to Sanofi/Bristol Myers Squibb clopidogrel litigation), the Baim Institute for Clinical Research (AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor-in-Chief, Harvard Heart Letter), the Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), CSL Behring (AHA lecture), Cowen and Company, the Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, the Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), WebMD (CME steering committees), and Wiley (steering committee); having been the Deputy Editor for Clinical Cardiology; being named on a patent for sotagliflozin assigned to Brigham and Women’s Hospital, who assigned to Lexicon (neither Bhatt nor Brigham and Women’s Hospital receive any income from this patent); having received research funding from Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Alnylam, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Otsuka, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, and 89Bio; having received royalties from Elsevier (Editor, Braunwald’s Heart Disease); and having been the site co-investigator for Cleerly.
- Ojha, Mohsin, Vavuranakis, and Khatri report no relevant conflicts of interest.





Comments