Could PET Imaging Be the ‘Game Changer’ for Detecting and Predicting Bioprosthetic Valve Degeneration?
Researchers are excited about 18F-fluoride uptake to detect early calcification and disease even before subclinical valve deterioration crops up on CT.
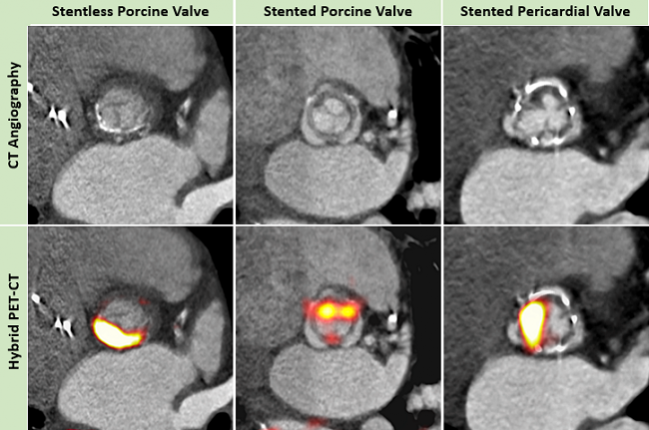
The use of 18F-fluoride positron emission tomography (PET)-computed tomography (CT) appears to be a more sensitive test than conventional assessments for the detection of subclinical valve degeneration following bioprosthetic aortic valve replacement, according to the results of a new study.
In the analysis, 18F-fluoride PET not only detected early bioprosthetic valve degeneration but also predicted new valvular dysfunction and overt valve failure.
“The durability of bioprosthetic valves is a really crucial issue with TAVR, and we don’t actually have any imaging techniques that can detect the early stages of these valves starting to fail and degenerate,” senior researcher Marc Dweck, MD, PhD (University of Edinburgh, Scotland), told TCTMD. “That was the idea going into this study, that we could use 18F-fluoride to detect early degeneration of the valves, and that’s really what our study has shown. Using the 18F-fluoride PET imaging technique, we can pick up degeneration of surgical valves before you see any sign of structural deterioration on CT or any signs of the valve starting to fail functionally on echocardiography.”
Lead investigator Timothy Cartlidge, MD (University of Edinburgh), agreed, telling TCTMD that 18F-fluoride PET has the potential to transform care for the increasing number of patients with bioprosthetic valves.
“For example, a routine PET-CT scan at 5 years post-valve implantation might help to differentiate patients at low versus high risk of valve failure and therefore guide appropriate clinical supervision and timely preparation for repeat intervention,” said Cartlidge. “18F-fluoride PET also holds promise in identifying novel therapeutic targets to retard the progression of valve degeneration and accelerate evaluation of new treatments and technologies.”
You can inject tracers targeting a specific biological process and see where in the body that disease process is active. Marc Dweck
18F-fluoride PET has been used to identify tissue calcification in a range of cardiovascular diseases, including aortic stenosis, abdominal aortic aneurysms, and high-risk or ruptured coronary atherosclerotic plaques. The advantage in using PETimaging for the detection of bioprosthetic valve deterioration is that 18F-fluoride binds to areas of developing microcalcification indicative of disease activity. In contrast, CT primarily quantifies areas of macrocalcification, say the researchers.
“You can inject tracers targeting a specific biological process and see where in the body that disease process is active,” said Dweck. “So the idea of 18F-fluoride PET is to measure calcification activity. CT tells you about calcium that has already formed, but the 18F-fluoride uptake tells you where the calcium is forming and is currently active.”
In the setting of bioprosthetic aortic valves, which predominantly break down via a process of calcification, the researchers hypothesized 18F-fluoride PET might be a particularly useful test for identifying and predicting valve degeneration.
Explanted Valve and Clinical Study
For their study published March 11, 2019, in the Journal of the American College of Cardiology, the researchers first performed an ex vivo assessment of degenerated bioprosthetic aortic valves explanted from 15 patients. CT detected leaflet calcification in 13 valves, which was confirmed in histology. Comparatively, all 15 valves showed signs of 18F-fluoride uptake that correlated with a range of histological markers of valve degeneration.
The clinical study was comprised of 78 subjects who had undergone previous surgical aortic valve replacement, including 7 with suspected bioprosthetic valve failure. In the 71 subjects without valve degeneration at baseline, 14 patients with abnormal CT findings showed signs of deterioration in valve function after 2 years, but there was no statistical difference in disease progression compared with those with normal CT.
In contrast, among the 24 subjects who showed evidence of increased 18F-fluoride uptake at baseline, there was “clear evidence of deteriorating bioprothesis function after 2 years” as assessed by the annualized change in peak transvalvular velocity, the researchers say. Overall, 18F-fluoride uptake correlated with deterioration in all conventional echocardiographic measures of valve function. For those with no evidence of 18F-fluoride uptake at baseline, there was no change in valve function at 2 years.
In total, 10 patients developed new bioprosthetic valve dysfunction during follow-up and all 10 had increased 18F-fluoride uptake at baseline (versus just five who had an abnormal baseline CT scan). Overall, 18F-fluoride uptake activity increased in a stepwise fashion across the progressive stages of valve dysfunction (all exhibited increased 18F-fluoride uptake at baseline) and 18F-fluoride was the only independent predictor of future bioprosthetic dysfunction.
“CT is a great technique for looking at native valves and the coronary arteries,” said Dweck. “But seeing the leaflets in bioprosthetic valves, and detecting structural changes, is quite challenging, particularly differentiating calcium in the leaflets versus the metallic struts that surround the valve. In our hands, trying to detect calcium with CT was challenging. It was much easier with PET because you get a very clear distinct signal.”
Calcification and Thrombus
The investigators say their results need to be validated, but Dweck is excited by the possibility of 18F-fluoride PET imaging for potentially identifying patients at risk for valve degeneration. One aspect of the study that was particularly intriguing was the link they observed between 18F-fluoride uptake indicative of calcification and thrombus on the valve leaflets. Following TAVR, thrombosis of the valve leaflets often occurs without hemodynamic consequences. In their study, those with leaflet thrombosis had high 18F-fluoride PET activity at baseline, said Dweck.
The study suggests it might be possible to identify valve degeneration early enough that it might be modifiable with medical therapy, added Dweck. For patients with CT-detected thrombus on the valve leaflets plus increased 18F-fluoride activity, “it’s quite a strong argument for giving that patient anticoagulation to try and get rid of the thrombus to prevent future valve degeneration,” said Dweck.
We’ve remained somewhat infatuated with acute and short-mid-term results and technical aspects of valve design and procedural technique. Rishi Puri
In an editorial, Zahi Fayad, PhD, and Claudia Calcagno, MD, PhD (Icahn School of Medicine at Mount Sinai, New York, NY), agree that the study has important implications “for the timely diagnosis and monitoring of subjects” with subclinical valve degeneration. Like Dweck, the editorialists suggest these patients may benefit for aggressive early interventions to prevent valve stenosis, regurgitation, and emergency valve replacements.
To TCTMD, Rishi Puri, MBBS, PhD (Cleveland Clinic, OH), who was not involved in the analysis, called the study a “game changer” for understanding how bioprosthetic valves behave and how they could potentially be monitored for accelerated degeneration. Also, 18F-fluoride PET could potentially be used to test and monitor the effects of novel therapies to attenuate the degeneration of the valves.
While there aren’t any therapeutic options if 18F-fluoride is detected on PET imaging, Puri said it’s early in the game, with researchers not yet having had the opportunity to test promising therapies such as PCSK9 inhibitors or investigational Lp(a)-lowering agents in this area. In the future, Puri said, bioprosthetic valve implantation will evolve where the procedure is coupled with optimal medical therapy, much like a patient receives a coronary stent and drug therapy to reduce the systemic burden of cardiovascular disease.
“However, the field hasn’t progressed to that point yet because we’ve remained somewhat infatuated with acute and short-to-mid-term results and technical aspects of valve design and procedural technique,” he said. “We need to now also move and focus on things beyond that. . . . The factors promoting both native aortic valve disease and bioprosthetic valve degeneration are systemic risk factors that can only be systemically modulated.”
Importantly, the field is shifting, too, with younger and younger patients receiving surgical and transcatheter bioprosthetic aortic valves. “So we need to start focusing on systemically protecting these valves and optimizing their life, and detecting those patients who may have risk factors for accelerated valve degeneration and conducting the right trials with novel agents to see if we change the natural history of bioprosthetic valves,” said Puri.
At present, though, it’s been difficult to convince drug companies to commit resources to fund studies using 18F-fluoride uptake on PET imaging as a biomarker to understand the effects of novel agents on valve mineralization and subclinical degeneration, according to Puri. Instead, pharmaceutical companies are focused on atherosclerosis and are slow to move beyond it.
“The valve field is exploding and we need to move with the times and convince pharma to assist those of us who’re trying to push the envelope to change the field,” said Puri.
Photo Credit: Tim Cartlidge
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Cartlidge TRG, Doris MK, Sellers SL, et al. Detection and prediction of bioprosthetic aortic valve degeneration. J Am Coll Cardiol. 2019;73:1107-1119
Fayad ZA, Calcagno C. Sodium fluoride PET and aortic bioprosthetic valve degeneration. J Am Coll Cardiol. 2019;73:1120-1122.
Disclosures
- Dweck, Fayad, Calcagno, and Puri report no relevant conflicts of interest.


Comments