DAPA-MI: Cardiometabolic Endpoints ‘Win’ With Dapagliflozin After Acute MI
(UPDATED) The trial had to switch out its hard primary endpoints for a win-ratio analysis: the result was a “soft positive,” one observer said.

PHILADELPHIA, PA— Dapagliflozin given to acute MI patients with either impaired LV function or Q-wave MI in the DAPA-MI trial significantly improved cardiometabolic outcomes, despite the trial enrolling a relatively low-risk population with no prior diagnosis of diabetes or chronic heart failure.
However, the addition of dapagliflozin (Farxiga; AstraZeneca) did not make a dent on cardiovascular death/heart failure hospitalizations, which was the trial’s original primary endpoint. Instead, based on very low mortality and hospitalization rates during the first 12 months, the trial was adapted to use a hierarchical win-ratio analysis using a mix of “hard” and “soft” clinical endpoints.
Stefan James, MD, PhD (Uppsala University, Sweden), presented the registry-based, randomized clinical trial here at the American Heart Association 2023 Scientific Sessions. The study, incorporating national clinical registry data from Sweden and the United Kingdom, was published simultaneously in NEJM Evidence, the monthly digital journal launched by the NEJM Group last year.
“Outcomes post-MI have improved over the last 20 years with the interventional cardiology and all the preventive medications, but that improvement has reached a sort of a plateau in in the last 5-6 years: there's still a risk of heart failure and cardiometabolic outcomes,” James told TCTMD. “We know we cannot improve it by more antithrombotic therapy, we cannot improve it much with more interventional procedures, but we can perhaps improve it by reducing the risk of developing heart failure, diabetes, hypertension, and other preventive measures.”
Prior trials of SGLT2 inhibitors, he noted, have typically required patients to be at least 3 months out from an MI. The DAPA-MI trial sought to reduce the risk of recurrent MI, heart failure, and mortality, while improving kidney function, body weight, lipid profiles by initiating the medication within the first 10 days of an acute MI, while maintaining other optimal medical therapies.
But the switch to the hierarchical win-ratio design will inevitably lead to some head-scratching: it’s not a design that many physicians—and indeed academics—are used to interpreting, said Michelle Kittleson, MD, PhD (Cedars-Sinai, Los Angeles, CA), who commented on the trial for TCTMD. “It makes you concerned that they modified the endpoint because they didn't meet the primary, and this post hoc assessment of endpoints always makes you concerned about how significant the results are, how valid the results are.”
Even James acknowledged, that the win-ratio endpoint posed a learning curve for DAPA-MI investigators, though he was ultimately struck by the fact that the design allows all patients to contribute endpoints across the spectrum of severity. “If they don’t die, they may have an MI, they may have atrial fibrillation, [they may have fewer] symptoms, or weight loss: [this is] information that would be lost in traditional analyses, but that is important to patients,” he said.
DAPA-MI Details
The trial enrolled 4,000 NSTEMI and STEMI patients with impaired LV systolic function or Q-wave MI, all of whom were hemodynamically stable within that 10-day window. They were randomized to either 10 mg of dapagliflozin on top of standard care, or to placebo. Follow-up occurred within the first 6-10 weeks postdischarge and again at 12 months.
The decision to switch to a hierarchical (win ratio) composite outcome, due to the low evert rates, was made in February 2023. The new endpoint combined seven components in hierarchical order: death, hospitalization for heart failure, MI, atrial fibrillation/flutter, type 2 diabetes, NYHA class, and weight decrease—all of which were included based on prior studies suggesting that dapagliflozin had positive effects on these domains. For the win-ratio analysis, matched pairs are created between the dapagliflozin and placebo arms, then each strategy is assigned a win or a loss per pair, starting with the topmost endpoint, death, then moving down the list sequentially if the previous event did not occur.
If we ask patients [what they want], of course they want to live, but they want to have a good life as well. Jonas Oldgren
Over approximately 12 months of follow-up, more “wins” were seen in the intervention group than in the placebo group: 32.9% versus 24.6% (win ratio 1.34; 95% CI 1.20-1.50). The wins continued to favor dapagliflozin when weight loss was removed from the analysis in the secondary endpoint.
But analyzed separately, cardiovascular death and hospitalizations for heart failure were nearly identical between the dapagliflozin and placebo groups: 50 versus 52 (2.5% vs 2.6%). There were no other significant differences across a host of individual “hard” endpoints. Instead, key drivers of the win ratio appeared to be new diagnoses of type 2 diabetes, which was more common in placebo-treated patients, and proportion of patients who lost at least 5% of their body weight between visits, which was greater among the dapagliflozin-treated patients.
With the hard event rates in this trial, at least at least 20,000 patients would have been needed to detect a statistically significant difference between groups, James noted. He also offered three theories as to why hard event rates were so low in the trial. The deliberate exclusion of patients with diabetes and heart failure was a key factor, he said, but given the evidence-based indications for the use of SGLT2 inhibitors in these patients, withholding them would have been unethical. The COVID-19 pandemic likely played a role—there were, at times, no hospital beds to be able to admit patients with heart failure hospitalizations, James said. Most of all, the excellent use of optimal medical therapy at discharge in both treatment groups was much better than anticipated at the trial outset.
Study chair Jonas Oldgren, MD, PhD (Uppsala University, Sweden), agreed in an interview, predicting that clinical trials will increasingly be designed and powered for hierarchical endpoints that include patient-centered outcomes. Traditional, hard-endpoint trials tend to have quality-of-life questionnaires as “sort of an add-on, in the supplement,” he explained. “Here we include them as part of the primary endpoint. . . . If we ask patients [what they want], of course they want to live, but they want to have a good life as well. So we believe these are clinically relevant endpoints, at least for the patients.”
Evidence-Based Indications
For now, on the basis of these data, Kittleson remains skeptical that adding dapagliflozin to the many meds patients are already started on after an acute MI will be worth the cost and added pill burden. “If we go straight to what was truly noteworthy here, a reduction in a new diagnosis of diabetes is noteworthy, although if you look at the actual absolute reduction, it's not that impressive. It's maybe a 2% absolute reduction,” she said. “Second, if you look at the change in weight, its maybe one kilogram [difference]. It’s a long run for a short slide. I'm not convinced this rises to the level of implementation, but it may, if they can better sort out—perhaps in a future study—a more homogenous patient population to truly understand the benefits.”
Muddying the waters, she elaborated, was the decision to combine both NSTEMI and STEMI patients, since the former made up more than one-quarter of the cohort, as well as the inclusion of patients with normal ejection fractions—approximately 20% of the cohort.
Also speaking with TCTMD, Carlos Santos-Gallego, MD, PhD (Icahn School of Medicine at Mount Sinai, New York, NY), pointed out that the cardiology community has seen another heart failure drug fall short of expectations in the post-MI setting, where today’s combination of technologies and medications are proving hard to beat. PARADISE MI, which tested sacubitril/valsartan (Entresto; Novartis) against an ACE inhibitor immediate after an MI, failed to show a reduction in cardiovascular deaths or MI. “Here we have the same situation,” Santos-Gallego said. “On the background therapy of optimal interventional techniques and good pharmacological therapy, dapagliflozin cannot add too much.”
In such a low-risk group, he added, it is difficult to predict whether any LV impairment will prove permanent. ICDs, he noted, are only implanted after a 40-day window post-MI, allowing clinicians to understand which patients will truly benefit from the intervention.
For me this is a positive trial, an undoubtedly positive trial, but I’d call it a ‘soft positive’ trial. Carlos Santos-Gallego
“Honestly for me this is a positive trial, an undoubtedly positive trial, but I’d call it a ‘soft positive’ trial,” he said. Asked whether he thinks dapagliflozin should gain an indication in patients without diabetes or heart failure who are recovering from an MI, Santos-Gallego said that, to him, the answer is still not clear. “I’m not so sure that these patients should receive SGLT2 inhibitors. However, I’m absolutely convinced that there will be indication creep and that these patients will end up receiving SGLT2 inhibitors, in the future,” he predicted
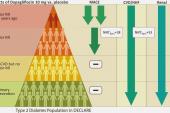
Oldgren stressed that DAPA-MI should be interpreted on top of other trials of SGLT2 inhibitors that have gone before and that prior studies can help hone treatment decisions. “For instance [from] DECLARE, we know that high-risk patients with diabetes and acute MI should definitely have this treatment,” he noted. From DAPA-MI, “we know they do not have to wait as long as they did in in DECLARE.”
Patients with other cardiometabolic risk factors, including those with prediabetes, should also be considered, particularly if they have impaired LV function, he added.
More insights will come from the EMPACT-MI trial of empagliflozin, which is also being conducted in acute MI but is enrolling patients at high risk for heart failure and includes individuals with diabetes. James also predicted that the impact of early initiation of dapagliflozin in this patient population will have secondary prevention benefits years out, even on top of the high-quality therapy these patients were already taking.
“Despite that, we were able to show a benefit on cardiometabolic outcomes, which in the long term I think can translate also into benefits on classical clinical hard endpoints as well,” James concluded.
Stephen Wiviott, MD (Brigham and Women’s Hospital, Boston, MA), the discussant following James’s late-breaking presentation, stressed the extent to which DAPA-MI was targeting unstudied patients—outside the universe of diabetes, heart failure, and chronic kidney disease where these drugs have truly proved their mettle. “From my standpoint,” said Wiviott, “the DAPA-MI trial does not suggest a new mandate to expand SGLT2 inhibitors to an isolated MI population without other SGLT2 inhibitor indications, but does support the safety of their use among patients with acute coronary syndromes.”
Importantly, he continued, “these results do not indicate a lack of clinical benefit in patients with prior MI and any of the diabetes, chronic HF, or CKD where SGLT2 inhibitors remain a pillar of guideline-directed medical therapy.”
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
James S. Dapagliflozin in myocardial infarction without diabetes or heart failure. Presented at: AHA 2023. November 11, 2023. Philadelphia, PA.
Disclosures
- James reports institutional research grants/supports from AstraZeneca, Novartis, Jansen, and Amgen.
- Wiviott reports research grants to the TIMI Study Group from Amgen, AstraZeneca, Janssen, Merck, and Pfizer, and personal consulting fees from Icon Clinical, Novo Nordisk, and Varian. His spouse is a current employee of Quotient Therapeutics (Flagship Labs) and a former employee of Merck.





Comments