New DECLARE-TIMI 58 Data Shine a Light on Diabetes Patients Most Likely to Benefit From Dapagliflozin
The analyses carving up the data according to prior MI and baseline EF are provocative and warrant the attention of cardiologists, experts say.
NEW ORLEANS, LA—Two new analyses from the DECLARE-TIMI 58 trial should serve as a reminder to cardiologists of the importance of prescribing SGLT2 inhibitors to reduce both atherosclerotic cardiovascular disease (ASCVD) and heart failure outcomes in diabetic patients at the highest risk for these events. And while these drugs are primarily used for glucose-lowering, this does not mean that endocrinologists need to shoulder the burden of making sure the right patients take these medications, experts told TCTMD.
“I’m not advocating that cardiologists manage blood glucose, but I think it's imperative that these drugs enter our routine arsenal, just like we saw with statins almost 30 years ago. These are cardiovascular medications and should be prescribed by cardiologists,” Darren McGuire, MD (UT Southwestern Medical Center, Dallas, TX), told TCTMD. “Endocrinologists can still use them to manage blood glucose, but we should be using them to modify cardiovascular clinical risk.”
McGuire is a co-author on two subanalyses from the DECLARE-TIMI 58 trial published last week in Circulation, one of which was also presented by lead author Eri Kato, MD, PhD (Kyoto University Hospital, Japan), as a late-breaking clinical trial last week at the American College of Cardiology (ACC) 2019 Scientific Session.
These are cardiovascular medications and should be prescribed by cardiologists. Darren McGuire
As previously reported by TCTMD, DECLARE-TIMI 58 demonstrated that the selective sodium glucose co-transporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga; AstraZeneca) in patients with type 2 diabetes and either high cardiovascular risk or established ASCVD reduced the risk of CV death or hospitalization for heart failure as compared with placebo. However, for MACE (CV death, MI, or ischemic stroke), the other efficacy endpoint in this trial, dapagliflozin was not superior to placebo.
Other research looking at SGLT2 inhibitors, as a class, have noted a more pronounced benefit of these agents among patients with prior MI, while two other major SGLT2 inhibitor trials—EMPA-REG Outcomes with empagliflozin (Jardiance; Boehringer Ingelheim/Eli Lilly) and CANVAS with canagliflozin (Invokana; Janssen)—have yielded different clues as to whether patients with pre-existing heart failure derive more benefit from the drug. These two populations of prior MI versus no prior MI and reduced EF versus preserved EF were the subject of the new subanalyses reported last week.
Dapagliflozin Impact by Ejection Fraction
Kato presented a post-hoc analysis of the DECLARE results looking at outcomes according to baseline ejection fraction, which was obtained in approximately one-third of the study participants at the outset of the study. Just 671 out of 17,160 patients were known to have an EF < 45% at the study outset, 1,316 had heart failure but preserved EF, and 15,173 had no history of heart failure.
As Kato showed, heart failure hospitalizations and cardiovascular deaths at 4 years were reduced by 38% in heart failure patients with reduced EF (HFrEF) compared with patients taking placebo. In heart failure patients with preserved EF at baseline (HFpEF), this reduction was less profound—just 12% compared with placebo (P for interaction = 0.046). Cardiovascular deaths were reduced by a whopping (and significant) 45% in the HFrEF patients taking dapagliflozin versus placebo, but this difference was not seen in HFpEF patients. Heart failure hospitalizations, meanwhile, were reduced with dapagliflozin versus placebo in both EF groups, although the relative and absolute reductions were more marked among patients with HFrEF than those with HFpEF.
“Patients with HFrEF are at the highest risk for CV events and mortality,” Kato concluded. “Treatment with dapagliflozin resulted in a lower rate of heart failure hospitalizations versus placebo in a broad spectrum of patients, including those with preserved ejection fraction.”
And while the profound effects on cardiovascular deaths were limited to HFrEF patients, Kato stressed that the effects in HFpEF patients should not be minimized. “I wanted to emphasize that dapagliflozin reduced hospitalizations for heart failure regardless of ejection fraction,” she said. “So although we did not see a mortality benefit in those without HFrEF, I think there is benefit to all patients.” This is particularly important given the dearth of effective therapies for improving CV outcomes in HFpEF patients, she added. “The fact that we saw a reduction in heart failure hospitalizations in HFpEF patients is important.”
Dapagliflozin Benefits According to MI History
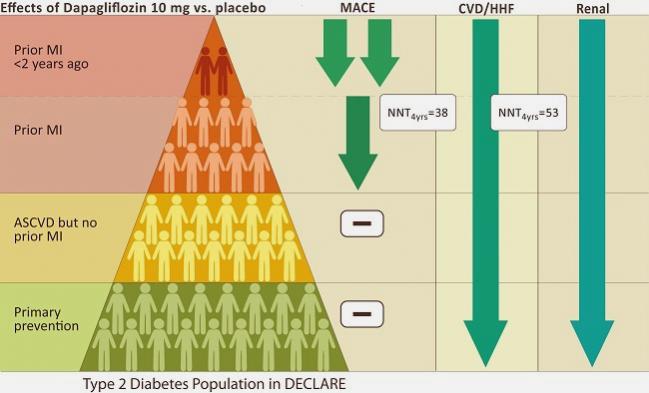
The second Circulation paper, published in tandem with Kato’s, was also a dichotomous analysis, but looked at MACE and CV death/heart failure hospitalizations according to patient history of MI. Led by Remo Furtado, MD, PhD (Brigham and Women’s Hospital, Boston, MA, and Universidade de Sao Paulo, Brazil), this analysis found that the 3,584 patients with prior MI in DECLARE experienced a significant 16% relative risk reduction in MACE (and an absolute risk reduction of 2.6%) as compared with placebo-treated patients. This reduction was not seen, however, among patients without prior MI. For the endpoint of CV death/HF hospitalizations, a relative risk reduction was seen in both the prior-MI and no-prior-MI groups, but the absolute risk reduction was significantly greater in the prior-MI group.
“Patients with type 2 diabetes mellitus and prior MI are at high risk of MACE and CV death/hospitalizations for heart failure,” Furtado and colleagues conclude. “Dapagliflozin appears to robustly reduce the risk of both composite outcomes in these patients. Future studies should aim to confirm the large clinical benefits with SGLT2 inhibitors we observed in patients with prior MI.”
Putting the Two Together
An editorial by Subodh Verma, MD, PhD (St Michael’s Hospital/University of Toronto, Canada), and John JV McMurray, MB, ChB, MD (University of Glasgow, Scotland), accompanying both papers attempts to put the findings from these two new analyses in perspective. To do so, they write, physicians need to understand that diabetes “adversely affects the pump (heart failure), pipes (atherosclerosis), and filter (renal disease), and that these effects can occur independently.”
In patients with diabetes who have had a prior MI, they continue, “the use of an SGLT2 inhibitor should be strongly considered as part of routine secondary prevention. In these individuals, SGLT2 inhibitors will reduce ischemic, heart failure, and renal events, benefits which appear to be mediated largely via glucose-independent mechanisms. The absolute risk reduction for MACE in post-MI patients are similar and additive to what are observed with antiplatelet therapies and intensive LDL-C lowering in recent trials.”
To TCTMD, Verma agreed that the two papers together “may be hard for people to get their heads around.” It may be helpful, he continued, to appreciate that both analyses are looking at distinct patient populations “across the spectrum of risk.”
The Furtado paper considers the spectrum of ASCVD in patients with diabetes—from at-risk, to established ASCVD, to post-MI—while the other considers the spectrum of heart failure: from no heart failure to HFpEF to HFrEF. For both analyses, Verma said, what is clear is that “the higher the risk, the greater seems to be the benefit of these agents, but the benefits seem to transcend, and are consistently seen, across the entire spectrum.”
Indeed, the editorial provides a helpful graphic to put this in perspective for the MI analysis (Verma provided TCTMD with similar graphic to accompany this story), but as Verma put it, this same “triangle of risk” could also have been used to illustrate the link between EF and outcomes. In other words, patients with diabetes and reduced EF would be at the top of the triangle, at the highest risk, while diabetic patients without heart failure would be at the base of the triangle—still benefiting from SGLT2 therapy, but to a lesser degree.
What both analyses should make clear to cardiologists is that diabetes is an essential tool for understanding risk at all stages of diseases, Verma said, and this holds true for both ASCVD and heart failure.
“Whether you are a general cardiologist, or an interventional cardiologist, or a cardiovascular surgeon, or a heart failure cardiologist, diabetes is a risk-stratifier irrespective of what stage patients are at,” Verma elaborated. “And it is also a determinant in many cases of how aggressive we are with therapy and how we treat people with certain interventions: bypass surgery versus angioplasty, for example.”
Verma, like McGuire, emphasized that these two analyses, on top of all the other SGLT2 inhibitor data make it imperative that cardiologists feel comfortable prescribing these agents in practice. Recommendations to do so have now been published by the ACC and the European Society of Cardiology and are incorporated in the new primary prevention guidelines, also released at the ACC meeting last week.
“All of these are directed to cardiologists, so I think they need to know that this science is solid, that the risk reduction is unprecedented in some cases, and that this is now becoming part of a primary prevention checklist,” Verma said. “The bottom line is that we need to get past the inertia of saying, ‘this is not my responsibility,’ and think about all of the strategies that we can embrace in primary and secondary prevention and add this to our list.”
The bottom line is that we need to get past the inertia of saying, ‘this is not my responsibility,’ and think about all of the strategies that we can embrace in primary and secondary prevention and add this to our list. Subodh Verma
McGuire, for his part, pointed out that the evidence supporting cardiovascular benefit has been “remarkably consistent” across this class of medications and, indeed, for the GPL1 receptor agonists as well, although he acknowledged that cardiologists may feel less comfortable with these agents, which are injectable therapies. Here, both Verma and McGuire stressed, cardiologists can work more closely with endocrinologists to make sure that the right patients are taking these agents.
Zeroing in on which patients will benefit most from an SGLT2 inhibitor was the aim of the two Circulation papers, reiterated McGuire, who is deputy editor at the journal. And while both analyses pointed to benefits “across the spectrum of disease,” they also single out patients in whom the drugs would yield the most benefit.
“It's also important to point out that these are really expensive medications, at least in the US,” McGuire observed. “In that context, I think it's important to figure out which subsets may have the greatest absolute benefits to make it most cost efficient, and that's what these two papers independently do, is use a dichotomous stratification by prior MI and by ejection fraction. What we see commonly is that effective therapies prove most effective in the highest risk patients, and we see that in both of these studies. You get more bang for your buck and more absolute risk reduction, meaning fewer needed to treat to derive a benefit, in HFrEF for heart failure-related outcomes and in prior MI for both ASCVD and heart failure-related outcomes.”
Beyond these latest findings remains the fact that these drugs were first established as safe and effective glucose-lowering agents intended to modify the risk of microvascular disease, and the latest analyses looking at cardiovascular endpoints have been welcome add-on findings, characterized by Verma and McMurray as a “serendipitous story.”
Nor has that story run its course. Still more trials are underway trying to tease out the glycemic effects from other drug benefits, based on the theory that SGLT2 inhibitors may yet prove beneficial for heart failure or ASCVD, even in patients with normal glucose.
Photo Credit: Courtesy of Subodh Verma, MD (adapted from the Circulation editorial by Verma and McMurray). Graphic Design: M. G. Rudakevich
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;Epub ahead of print.
Furtado RHM, Bonaca MP, Raz I, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes and prior myocardial infarction: a sub-analysis from DECLARE TIMI-58 trial. Circulation. 2019;Epub ahead of print.
Verma S, McMurray JJV. The serendipitous story of SGLT2 inhibitors in heart failure: new insights from DECLARE-TIMI 58. Circulation. 2019;Epub ahead of print.
Disclosures
- Kato reports receiving personal fees from Daiichi Sankyo; grants and personal fees from Ono Pharmaceutical; and personal fees from AstraZeneca, Bristol-Myers Squibb, and Tanabe-Mitsubishi Pharma outside the submitted work.
- Furtado reports receiving grant support from the Lemann Foundation Cardiovascular Research Postdoctoral Fellowship, Harvard University/Brigham and Women’s Hospital; honoraria from AstraZenaca; and grants (received from his institution) from AstraZeneca, DalCor, Boehringer, Pfizer, Janssen, and Sanofi, outside the submitted work within the last 36 months.
- Verma reports holding a Tier 1 Canada Research Chair in Cardiovascular Surgery; receiving research grants and/or speaking honoraria from Amgen, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novartis, Novo Nordisk, Sanofi, Servier, and Valeant; serving as a member of the scientific excellence committee of the EMPEROR-Preserved and EMPEROR-Reduced trials, the scientific committee of the DETERMINE-A and DETERMINE-B trials, and the global expert panel of the SELECT study; and is a national lead investigator of the Dapa-HF, DELIVER, DETERMINE-A, DETERMINE-B, EMPEROR-Preserved, EMPEROR-Reduced, SELECT, and SOLOIST studies.
- McMurray reports that his employer, the University of Glasgow, paid for his participation in clinical trial committees by AbbVie, AstraZeneca, Amgen, Bayer, Bristol-Myers Squibb, Dalcor, GlaxoSmithKline, Merck, Novartis, Resverlogix, Stealth and Theracos. In addition, his travel and accommodation costs for attendance at meetings related to some of the clinical trials have been funded by these sponsors; also, that McMurray’s employer has also paid for his attendance at advisory boards organized by Novartis and Sanofi-Aventis.


Comments