DCBs, Stents, and ‘Swirling Flow’ Show Promise in Femoropopliteal Disease
In late-breaking trial presentations at VIVA 2017, researchers outlined directions the field is taking to combat even the most challenging PAD cases.

LAS VEGAS, NV—Drug-coated balloons (DCBs) and unique stent platforms seem poised to supplant angioplasty as the go-to treatment for femoropopliteal disease. In late-breaking trial presentations here at VIVA 2017, researchers shared long-term data for these interventions, with encouraging outcomes in some of the most challenging patient populations.
Presenting 4-year results of the IN.PACT SFA trial, Peter Schneider, MD (Kaiser Foundation Hospital, Honolulu, HI), noted that while DCBs have entered into the treatment paradigm for PAD, “we’re grappling with where it’s going to land.” Long-term follow-up of these devices, particularly with regard to safety, he added, is limited.
The data from the multinational trial, which randomized 331 patients to the IN.PACT Admiral (Medtronic) paclitaxel-coated DCB or PTA, showed approximately 25% greater patency with the intervention and a higher freedom from clinically driven TLR (76.8% vs 70.4%; log-rank P = 0.0399) at 4 years. Additionally, time to reintervention in the DCB group was more than double that in the PTA group (739.2 vs 302.9 days; P < 0.001). There were no device- or procedure-related deaths and no major target-limb amputations in either group during follow-up.
Panelist John Kaufman, MD (Oregon Health & Science University, Portland, OR), said he was struck by the “modest TLR” difference, adding that he would have expected a much wider gap between the DCB and PTA groups.
But Schneider suggested that the time to reintervention may be the more important factor to consider, since much of the focus of PAD interventions is to extend that time beyond the traditional 1-year mark that has been associated in the past with PTA and BMS.
Although it’s clear that this [intervention] doesn’t last forever . . . we do see some sustained benefit. Peter Schneider
“What we’re doing now is we’re pushing that peak of restenosis further and further out,” he said. “For someone who comes in my office today, I want to push that peak of restenosis as far out into the future as I can. And so although it’s clear that this [intervention] doesn’t last forever . . . we do see some sustained benefit.”
Challenges in the Real World
Moving beyond the trial population, Thomas Zeller, MD, PhD (Universitäts-Herzzentrum Freiburg-Bad Krozingen, Germany), presented 2-year data on the same DCB in a challenging real-world cohort enrolled in the IN.PACT Global study that included patients with lesions longer than 15 cm, chronic total occlusions, in-stent restenosis, and diabetic patients.
Among the 1,406 patients treated, freedom from clinically driven TLR was 83.3% through 2 years. Primary sustained clinical improvement was 68.6%, and the primary safety composite (freedom from device- and procedure-related death within 30 days, freedom from target limb amputation within 2 years, and freedom from clinically driven TLR within 2 years) was 81.7%. Referring to the safety composite, Zeller said: “For a balloon-based interventional approach, that’s a very good outcome result.”
Compared with the IN.PACT SFA trial cohort, the Global patients had an approximately 7% higher intervention rate after accounting for the most challenging lesions.
A third late-breaking trial also focused on broadening interventions to real-world patients, but through the use of a DES rather than a DCB. For the Zilver PTX postmarket study in Japan, researchers led by Michael Dake, MD (Stanford University School of Medicine, CA), looked at 4-year outcomes in the first 900 patients treated with the paclitaxel-eluting Zilver PTX (Cook Medical). Unlike prior studies of the device, the Japan study was an all-comers population with no exclusion criteria.
Freedom from TLR remained consisted through the follow-up period despite the burden of more complex lesions, topping out at 78.3% at 4 years and with a clinical benefit (freedom from amputation and TLR) at the same time point of 72.7%.
In subgroup analyses, rates of TLR were similar between patients with and without renal failure. Additionally, there was no difference in the amputation rates among the 7% of patients with no patent runoff vessels versus those with runoff vessels, although there was a higher incidence of critical limb ischemia in the no-runoff group. Among those with in-stent restenosis, freedom from TLR was slightly lower compared with patients without in-stent restenosis, but the difference fell short of statistical significance (P = 0.05).
Changing Blood Flow to Combat Restenosis
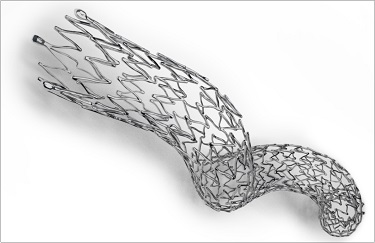
A new type of stent could surmount some of the obstacles presented by complex femoropopliteal lesions. Zeller presented post hoc data from the MIMICs study, which assessed an investigational nitinol device that looks, from a distance, like a child’s Slinky toy. Instead of being a straight stent, the BioMimics 3D helical stent (Veryan Medical) is curved to mimic the natural geometry of the vascular system. The stent enables what Zeller described as a “swirling flow” of blood. In a press conference prior to his presentation, he said the swirling flow provides a vascular benefit by protecting against de novo stenosis and restenosis.
The main MIMICS trial randomized 76 patients with symptomatic PAD to the helical stent or a straight control stent. For the post hoc analysis, Zeller and colleagues looked at how the stent performed in the presence of confounders such as calcification, long lesion length, CTO, and diabetes. Overall, primary patency was unaffected by any of the confounders, and presence of calcification did not affect curvature of the stent or the generation of the swirling flow inside the stent.
Panelist Krishna J. Rocha-Singh, MD (Prairie Vascular Institute, Springfield, IL), questioned how the atypical nature of the device would affect how regulatory agencies might evaluate it in comparison to other stents for this indication.
“It really does depend on each individual stenting technology,” said panelist Donna Buckley, MD (Division of Cardiovascular Devices, US Food and Drug Administration). “We have a lot of information right now in the SFA for stents, and I think we’re generally able to build on a lot of that by experience, but it really does depend on the novelty of the technology.”
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Schneider P. Four-year results of the IN.PACT SFA trial. Presented at: VIVA 2017. September 12, 2017. Las Vegas, NV.
Zeller T. Two-year results from the IN.PACT Global study. Presented at: VIVA 2017. September 12, 2017. Las Vegas, NV.
Dake M. Insights into the ZILVER PTX study in Japan. Presented at: VIVA 2017. September 12, 2017. Las Vegas, NV.
Zeller T. Primary patency of a 3D helical stent: significant outcomes from the MIMICS study. Presented at: VIVA 2017. September 12, 2017. Las Vegas, NV.
Disclosures
- Zeller reports honoraria from 480 biomedical, Abbott Vascular, Biotronik, Boston Scientific Corp, Cordis, Gore & Associates, Medtronic, Shockwave Medical, Spectranetics, Veryan/Novate, Volcano, and WL Gore as well as consulting fees from Boston Scientific, Medtronic, Gore & Associates, Spectranetics, and Veryan/Novate.
- Dake reports honoraria from Bard Peripheral Vascular, Cook Medical, and Gore & Associates and consulting fees from Cook Medical, Gore & Associates, and Novate.
- Schneider reports consulting fees from Cagent, Cook Medical, and Intact Vascular.


Comments