‘Enormous’ QoL Improvements With Mavacamten in HCM
Better health status is what matters to these patients, John Spertus says. Here, when the drug was stopped, they felt worse.
Within weeks of starting therapy, patients taking mavacamten for symptomatic obstructive hypertrophic cardiomyopathy (HCM) report large gains in health quality with a number needed to treat (NNT) of just five to see large to very large improvements in patient-reported quality-of-life scores.
Tellingly, the improvements in Kansas City Cardiomyopathy Questionnaire (KCCQ) regress abruptly with treatment withdrawal, said John Spertus, MD (Saint Luke’s Mid America Heart Institute, Kansas City, MO), who presented the health status analysis from EXPLORER-HCM at the American College of Cardiology 2021 Scientific Session today.
Mavacamten (Bristol Myers Squibb), an investigational first-in-class cardiac myosin inhibitor, has been designated as a breakthrough therapy by the US Food and Drug Administration, allowing for expedited development and review process. Primary results from EXPLORER-HCM, first released at the European Society of Cardiology 2020 meeting and reported by TCTMD, demonstrated that in patients with HCM, mavacamten on top of medical therapy significantly improves hemodynamics, functional capacity, and symptoms when compared with placebo. The primary endpoint, based on two different combinations of peak VO2 improvement and NYHA improvement or stabilization, was more than doubled in the mavacamten group versus the placebo group.
“To me, we've got the tail wagging the dog, where we’re focusing on these surrogate outcomes and not on what really matters to patients,” he said. “[This] is why I built the KCCQ long ago, to kind of focus on what’s important to patients and that's what these data show.”
New Avenues Explored
As previously reported, EXPLORER-HCM randomized 251 patients (mean age 58.5 years) with obstructive HCM to mavacamten or placebo for 30 weeks. Patients were started on a 5-mg daily dose of mavacamten, which was uptitrated at weeks 8 and 14. Most patients had NYHA class II symptoms at baseline, and nearly all patients were on background medical therapy with beta-blockers or a calcium channel antagonist. In addition to meeting its primary endpoint, the trial also demonstrated that significantly more patients randomized to mavacamten met the higher benchmark of peak exercise capacity, showed improved postexercise left ventricular outflow tract gradient, peak VO2, and NYHA class from baseline to week 30, as well as improvement in two validated HCM symptom scores.
The health status analysis that Spertus presented today delves deeper into patient-centric endpoints, based on KCCQ data obtained at baseline and at 6, 12, 18, 30, and 38 weeks—30 weeks being the end of the treatment and 38 weeks being the end of the study.
As Spertus showed, the mean change in KCCQ Overall Summary Score at 30 weeks was 9.1 points greater in the mavacamten group than in the placebo group (95% CI 5.5-12.8). Putting that in perspective, “this is one of the largest mean differences of any medication,” he said during his talk. Elaborating to TCTMD, Spertus observed that while the sodium-glucose cotransporter 2 inhibitors have been shown to help improve symptoms, function, and quality of life “a lot,” the average benefit across trials is “less than third of what was seen in this trial population, so a really big benefit.”
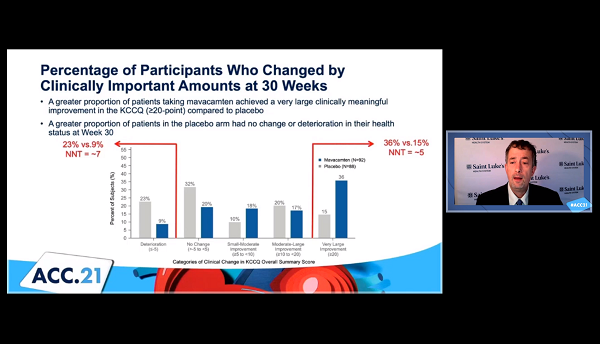
Moreover, in an analysis that attempted to look closer at individual-level benefits, a greater proportion of patients taking the study drug achieved a “very large” and clinically meaningful improvement of 20 points or greater, compared to placebo (36% vs 15%; NNT = 5), whereas a higher proportion of placebo-treated patients had no change or a deterioration in their health status (23% vs 9%; NNT = 7).
Drug Stopped, Health Scores Dropped
Strikingly, health status scores “regressed rapidly” in the 8 weeks after the study drug was stopped, dipping below the levels seen in the placebo group. By comparison, Spertus noted, device trials in structural heart disease using TAVI or leaflet coaptation for mitral regurgitation have shown even bigger gains in quality of life. “What we've never been able to do with TAVR or with MitraClip is: we can take away the treatment, we can take away the drug and see that all that benefit goes away, and I think that's what’s remarkable here.”
Devices, unlike many drugs, can have that effect because they’re acting directly on the pathophysiology causing the problem and symptoms, Spertus noted. Mavacamten appears to be doing something similar, although the mechanisms for that need to be further evaluated.
FDA Approval and Price
Spertus hopes that, assuming the FDA approves the drug, the palpable and profound impact on patient quality of life will boost drug compliance. “They won't have problems with compliance, because if people stop taking it, they will feel worse,” he predicted to TCTMD.
But this also means that the drug’s benefits will likely be confined to patients whose disease is having a substantial impact on their quality of life. This is not too different from what was seen in the ISCHEMIA trial, he noted: “If you have no symptoms you can't feel better, so if we start expanding [mavacamten] into an asymptomatic population of patients, then the quality-of-life benefits are no longer going to be apparent.”
That raises the specter of just how much the manufacturer will end up charging for mavacamten if the potential patient population is small.
It’s not guaranteed to work, but I think for a substantial proportion of patients they got an awful lot better. John Spertus
“That's concerning to me,” Spertus acknowledged. “I don't know how they're going to price it. The patients with obstructive hypertrophic cardiomyopathy that's this symptomatic is not a [large] market, so it might be very, very expensive.” One possibility worth exploring, he said, is whether the benefits of this drug are due to better left ventricular relaxation; if that’s the case, “that would open up the HFpEF market, which is enormous.”
Matthew Martinez, MD (Morristown Medical Center, NJ), discussing the results following their presentation today, asked whether Spertus envisioned mavacamten as additive to other “medical treatments in the toolbox” or more-invasive surgical approaches to treating HCM, or as a replacement.
Spertus, in response, pointed out that this is the largest trial to date in patients with hypertrophic, obstructive cardiomyopathy and the first to have such a “rigorous” assessment of the therapy’s impact on health status.
“Certainly I think before offering an irreversible surgical or percutaneous intervention to decrease the bulk of the septum, I would want to try this, as tolerated by the patients. It’s reversible, and you can always reserve the surgical option—you can’t undo an alcohol septal ablation or undo myectomy—so from my perspective, I think this would be something that I would reach for early,” he said. “If patients are not responders, then I would be trying other [medical] options. . . . And if all of those fail, I would be pursuing invasive options. It’s not guaranteed to work, but I think for a substantial proportion of patients they got an awful lot better.”
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Spertus J. Health status benefits of mavacamten in patients with symptomatic obstructive hypertrophic cardiomyopathy: results from the EXPLORER-HCM randomized clinical trial. Presented at: ACC 2021. May 15, 2021.
Disclosures
- EXPLORER-HCM was funded by MyoKardia.
- Spertus reports consulting for Abbott, Amgen, Bayer, Janssen Pharmaceuticals, Merck, MyoKardia, Novartis, and UnitedHealthcare; grant support from Abbott Vascular; holding the copyright to the SAQ, KCCQ, and PAQ; and being a member of the board of directors for Blue Cross Blue Shield of Kansas City.





Comments