Faster, On-site FFRCT May Be on the Horizon Through Innovation and Collaboration
Manufacturers and researchers made several announcements this week in conjunction with SCCT 2017 that could change the way FFRCT is used.
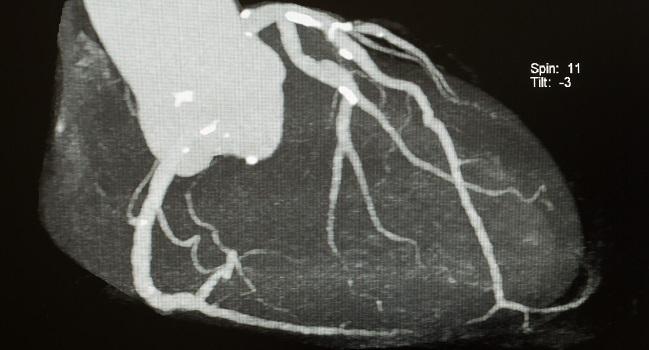
WASHINGTON, DC—A relative newcomer to the field of cardiac imaging, noninvasive fractional flow reserve derived from computed tomography (FFRCT) has been drawing lots of attention this week from cardiac imaging specialists attending the Society of Cardiovascular Computed Tomography (SCCT) 2017 Annual Scientific Meeting.
Several studies have suggested that FFRCT is better than CT angiography alone at identifying clinically significant stenosis and “can hopefully prevent people with non-flow-limiting disease from coming to the cath lab,” said Hasan Jilaihawi, MD (NYU Langone Medical Center, New York), who outlined the “gatekeeper” role for this technology Friday at SCCT 2017. The main drawback of this algorithm-based technology is that, for now, it can only be calculated off-site by a third-party company, HeartFlow, which has a turnaround time ranging from hours to days.
But just this week, HeartFlow and GE Healthcare announced a new relationship “with the goal of increasing the clinical availability and adoption” of the technology. Many hope this kind of collaboration, which HeartFlow also formed with Siemens back in March, could one day allow the technology for calculating FFRCT to be built directly into the scanners, permitting real-time analysis of suspected CAD. The current plan, however, is not to have any technology built into scanners but rather to allow for cloud-to-cloud connection between GE’s and HeartFlow’s software clouds, a HeartFlow representative clarified.
Another budding avenue for FFRCT would be a novel on-site estimation of fractional flow reserve using patient-specific lump parameter models, a concept also discussed at SCCT 2017. On Friday, Robbert Van Hamersvelt, MD (University Medical Center Utrecht, the Netherlands), presented results from an ongoing retrospective study of a prototype software tested among 25 eligible patients (39 vessels) with suspected CAD who underwent both invasive FFR and CTA, with FFRCT calculated while blinded to the FFR results.
According to Van Hamersvelt, the software identified 46% fewer false positives compared with coronary CTA with a computation time of less than 10 seconds. Compared with coronary CTA, their version of FFRCT had similar sensitivity, but increased specificity, positive predictive value, negative predictive value, and accuracy at identifying stenoses of 50% or greater.
“We conclude that this rapid on-site FFRCT prototype has the potential to improve the diagnostic accuracy of coronary CTA for the detection of hemodynamically significant stenosis and, could thereby prevent patients from unnecessary invasive [coronary angiography],” Van Hamersvelt said in his closing remarks.
Speaking with TCTMD, he said that having instant feedback from fast, on-site FFRCT might not matter in many stable CAD cases, but for others it will aid the clinical workflow particularly “if you want to change something to the segmentations or if you want to do stent planning.”
Van Hamersvelt said his team’s plan is to follow through with this proof-of-concept study and ultimately plan a prospective analysis comparing the prototype to traditional invasive FFR.
As for collaborations between HeartFlow and scanner manufacturing companies like Siemens and GE Healthcare, Van Hamersvelt said these could “really work out in a positive way, as good image quality is requisite and could improve the FFRCT outcome. On the other hand, I hope HeartFlow stays developing their software on all vendor platforms to be able to serve as many patients as possible, as this could potentially prevent a lot of patients from unnecessarily going into the cath lab, like we have seen in all the recent studies.”
Also speaking with TCTMD, Jilaihawi agreed that partnerships like these “will increase the accessibility to FFRCT technology in the United States and facilitate a better understanding of its role in clinical practice.”
In another announcement, HeartFlow said this week that it earned Category III Current Procedural Terminology (CPT) codes from the American Medical Association, which will help with reimbursement for FFRCT. “This is important because this is not a cheap analysis. It costs about $1,500 currently,” Jilaihawi said in his presentation. “But the caveat is, how do you use this in practice? . . . Its exact role in patient workflow requires further study.”
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Jilaihawi H. CTA and CT-FFR as the gatekeeper to the cath lab. Presented at: SCCT 2017. July 7, 2017. Washington, DC.
Van Hamersvelt R. Initial experience with a rapid non-invasive on-site estimation of CT fractional flow reserve. Presented at: SCCT 2017. July 7, 2017. Washington, DC.
Disclosures
- Jiliaihawi reports serving as a consultant to Edwards Lifesciences and Venus Medtech and receiving research support from Medtronic, Abbott, and St. Jude.
- Van Hamersvelt reports no relevant conflicts of interest.


Comments