FDA: All AFX Endovascular AAA Systems Now Under Class I Recall
Continued reports of serious endoleaks after EVAR have prompted a broadening of a recall originally specific to AFX devices with Strata.
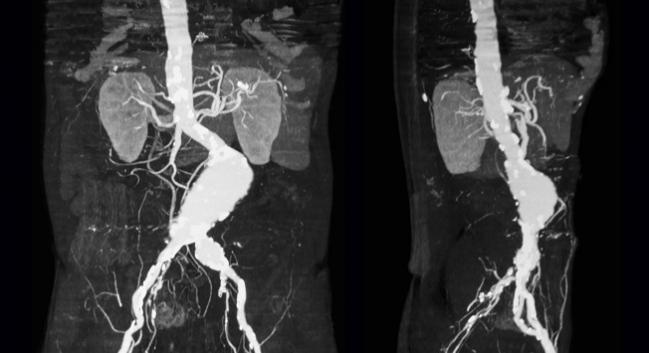
The US Food and Drug Administration (FDA) has issued a MedWatch recall notice for all AFX Endovascular AAA Systems by Endologix following a voluntary recall action by the company in July. The notice, announced yesterday, designates the action as a Class I recall, the most serious type.
The FDA communication comes 1 year after the agency issued a warning of rising rates of endoleak after EVAR, and 4 months after concluding that the phenomenon was specific to the AFX with Strata device manufactured by Endologix.
In the MedWatch notice, the FDA said: “It is important to note that although this recall applies to all AFX Endovascular AAA Systems, most reports of endoleaks have concerned the AFX with Strata graft material.”
After AFX with Strata was recalled, Endologix (Irvine, CA) changed the graft material to an improved material known as Duraply. In a safety letter to physicians issued in July 2018, Endologix said it “has been monitoring the effectiveness of these changes through its complaint monitoring system” and maintained that “the reported Type IIIa and IIIb endoleak estimated complaint rates at equivalent time points past 1 year have been lower for the AFX System with Duraply and AFX2 System with Duraply compared to the AFX System with Strata . . .”
Just 1 month prior to the letter, TCTMD reported that the FDA had concluded after an investigation that the rise in cases being reported through the Medical Device Reporting (MDR) system database, and initially thought to be attributed to various endovascular graft device models on the market, appeared to be specific to Endologix AFX with Strata.
They also note that the AFX with Duraply and AFX2 devices “have been distributed for a shorter time and it is unclear if these devices have fewer endoleaks or if they have not been implanted long enough for endoleaks to occur.”
The AFX System recall involves 61,300 devices manufactured from March 2011 to the present. In addition to close monitoring and follow-up of patients who have been implanted with these devices, Endologix and the FDA recommend considering the possibility of type III endoleaks in patients presenting with symptoms of potential aneurysm expansion or rupture as well as discussing all available treatment options for type III endoleaks, including the risks and benefits of each.
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
US Food and Drug Administration. Endologix, Inc. recalls AFX endovascular AAA systems due to risk of type III endoleaks. Published on: October 15, 2018. Accessed on: October 15, 2018.


Comments