FFRCT Shifts Patient Management, Linked to Better 90-Day Outcomes: ADVANCE Registry
Though the study isn’t definitive, “that doesn’t mean these aren’t important and interesting data,” one FFRCT expert says.
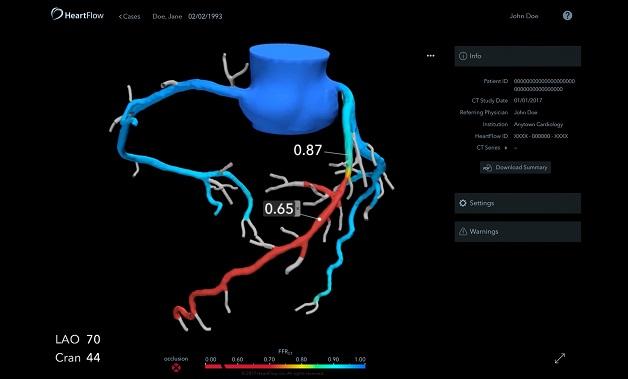
Using coronary CT angiography (CTA) to derive fractional flow reserve (FFR) values in patients with stable chest pain leads clinicians to rethink their management plans two-thirds of the time compared to CTA alone, according to data from the ADVANCE Registry released last week. Additionally, patients whose FFRCT values exceeded 0.80 were less likely to proceed to invasive angiography or undergo revascularization than cases informed by CTA alone, and they were less likely to die or experience MI within 90 days.
“It’s a real-world registry study, so looking at real-world practice,” study investigator Timothy A. Fairbairn, MBChB, PhD (Liverpool Heart and Chest Hospital, England), told TCTMD. Based on the findings, FFRCT seems to be a “helpful test that is being utilized,” he said.
This is especially true for cases in which the treating physician was initially uncertain about the significance of disease, Fairbairn commented. Also important is the 22.3% of patients whose coronary CTA indicated that an invasive angiogram, and potentially revascularization, was needed. Among them, nearly one-quarter were safely switched to medical therapy.
“Therefore, we’re safely deferring individuals who might have gone ahead and had maybe an inappropriate and invasive angiography. So the test is being used in a sensible, practical manner, and it does really seem to be benefiting . . . in terms of moving patients in the right direction,” Fairbairn said.
The results were presented in a late-breaking session at the European Society of Cardiology Congress 2018 and simultaneously published online in the European Heart Journal.
In the days after the presentation, debate emerged over ADVANCE’s methodology and whether it undermined the strength of its findings.
Commenting on the study for TCTMD, Pamela Douglas, MD (Duke University, Durham, NC), said that with a registry, of course there would be some methodological issues. “It’s real world, it’s not even a tightly controlled observational study, and it’s certainly not a randomized trial,” she said. “That doesn’t mean these aren’t important and interesting data. It does mean that they are not definitive, which is fine. It just means that we need to do the definitive trial, but this provides us with a lot of evidence to properly design and construct that trial, power it, and so on.”
That process is already underway in the PRECISE trial, for which she is principal investigator, and the DECISION trial, she said. Douglas acknowledged that both are funded by HeartFlow, as is the current registry.
Subha Raman, MD (Ohio State University Wexner Medical Center, Columbus), speaking with TCTMD, also said the study design isn’t optimal. Considering the length of time “this technology has been out there with such anticipation and such promise, I feel like we’re still waiting for a randomized trial to get the type of evidence that we need to make recommendations that FFRCT is helpful in evaluating patients with stable chest pain and suspected coronary disease,” she commented.
Writing in an accompanying editorial, Todd C. Villines, MD (Walter Reed National Military Medical Center, Bethesda, MD), expresses some skepticism, calling the study’s primary endpoint of reclassification in treatment strategy “a simulation exercise that is not reflective of guidelines or routine clinical care.” He also points out that studies of the technology have almost exclusively been sponsored or performed by investigators supported by the manufacturer.
Still, “the current results represent a much-needed, real-world assessment of this exciting disruptive technology,” he says.
The ADVANCE Registry
Fairbairn and colleagues analyzed treatment plans and outcomes for 5,083 patients with clinically suspected CAD and ≥ 30% degree stenosis on coronary CTA who were enrolled at 38 sites in Europe, North America, and Japan between July 15, 2015, and October 20, 2017. More than half of the cohort (58%) presented predominately with angina.
Site investigators first described their management plan and treatment strategy based on CTA alone. Then, at physician discretion, patients with stenoses in the range of 30% to 90% on CTA could be evaluated using FFRCT (HeartFlow), after which investigators were given the option of rethinking their plan. Nearly two-thirds of the time (63.5%) they did so. For the primary endpoint, reclassification between a core lab-determined strategy based on CTA alone versus CTA plus FFRCT, the rate was 66.9%.
CTA detected coronary atheroma of ≥ 50% in 72.1% of patients and of ≥ 70% in 32%. Slightly more than one-quarter had two-vessel disease and 9.4% had triple-vessel disease. Ischemia was indicated by an FFRCT value ≤ 0.80 in at least one coronary territory for 61.9% of patients.
When clinicians’ management decisions were informed by FFRCT, the need for additional testing dropped from 57.9% to 2.9%, while greater emphasis was placed on medical therapy (increasing from 19.2% to 63.5%) and PCI (increasing from 20.4% to 30.5%). The proportion of cases slated for CABG held steady at around 3%. But looking at actual management, most patients (75.4%) ultimately received medical therapy alone within the first 90 days, with PCI performed in 21.4% and CABG in 3.1%.
With FFRCT, 61.9% of patients had values ≤ 0.80, while only 34.4% of sites recommended them for angiography. This is true even though seven in 10 of patients in this FFRCT group had > 50% diameter stenosis on CTA, meaning that clinicians are making choices based on other things like patient characteristics, comorbidities, symptoms, and anatomy, Fairbairn said. Fully 72.3% of patients who had an FFRCT value ≤ 0.80 and underwent invasive angiography were then revascularized.
In patients with FFRCT values > 0.80, no death/MI occurred within 90 days, but in patients with values ≤ 0.80, there were four MIs and 10 deaths, for a combined rate of 0.3% (HR 14.68; 95% CI 0.88-246.00). In all, the FFRCT ≤ 0.80 group had a MACE rate of 0.6% when including five unplanned hospitalizations for ACS and urgent revascularization (HR 19.75; 95% CI 1.19-326.00).
Aiming for Diagnostic Certainty
“We’re really able to use this technology to make sure that patients are reassured, that physicians are reassured and have more diagnostic certainty, and that patients are safely deferred from having invasive strategies that may not be necessary,” Fairbairn said, adding that long-term follow-up planned at 1 and 3 years will be key.
Douglas agreed with the need for long-term follow-up, though she said most of the difference in events would likely have occurred within 90 days. Another interesting aspect is that 24.3% of patients were asymptomatic, though that is unfortunately representative of the population being tested for CAD around the world, she said.
Notably, though, only 3.2% of CTAs that were submitted for FFRCT analysis were rejected because of image quality, Douglas pointed out. This “basically says this tool is ready for prime time. There have been a lot of questions with PLATFORM and some other studies that over 10% were not analyzable. I think our CT scans have cleaned up and the algorithm has been cleaned up and it is ready for routine use.”
Asked by TCTMD whether the centers participating in ADVANCE were representative of who’s using FFRCT today, Fairbairn said that the study began when much fewer sites employed the technology. For example, the three ADVANCE centers in the United Kingdom were the only ones in the country to do FFR at the time, but by the end of 2018, it’s predicted that there will be a total of 28 to 30 centers. Based on his experience training new users, however, Fairbairn said that FFRCT-based management patterns are likely to be consistent.
“What’s important to know,” Douglas concluded, “is FFRCT is not going to tell you everything about lesions, but what it is going to tell you is [who] you can safely manage medically. We always want our tests to create a positive diagnosis, so it’s a little hard to swallow a test that says you don’t need to do something or you don’t have obstructive disease. But that’s equally important to patients in terms of care.”
Raman, though, described FFRCT as a “tough sell” at this point, “because of the type of evidence that’s in the literature. We hold the bar pretty high when we’re asking clinicians to change practice. And when we look at what other modalities have done to reach that bar, it would seem reasonable to expect the champions of this technology to do the same. The patients we serve really deserve that to say, ‘Okay, I understand we’re going in a new direction in terms of the testing that you’re recommending for me.’”
Cost also matters, she said. According to Villines’ editorial, FFR increases the price of the overall CT study by more than fourfold.
“Now we have a number. It’s a concern,” Raman agreed. “With imaging always in the headlights of those who scrutinize healthcare expenditure, we need some really solid evidence that a multiplicative increase in the cost of coronary CT has a considerable return on investment, for both patients and payers.”
Douglas predicted that if FFRCT does safely reduce unnecessary testing and procedures, its cost will easily be covered. Villines, for his part, called for “independent studies using measured costs” as well as improved selection criteria for targeting which patients are likely to benefit from FFRCT.
Photo Credit: HeartFlow
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Fairbairn TA, Nieman K, Akasaka T, et al. Real-world clinical utility and impact on clinical decision-making of coronary computed tomography angiography-derived fractional flow reserve: lessons from the ADVANCE Registry. Eur Heart J. 2018;Epub ahead of print.
Villines TC. Can CT-derived FFR better inform clinical decision-making and improve outcomes in stable ischaemic heart disease? Eur Heart J. 2018;Epub ahead of print
Disclosures
- Fairbairn reports being on the speaker’s bureau for HeartFlow.
- Douglas reports receiving research funding from HeartFlow.
- Raman reports no relevant conflicts of interest.
- Villines reports being the immediate past president of the Society of Cardiovascular CT and the current chair of the ACC Imaging Council.


Comments