Good Results With Next-Gen, Self-Expanding TAVR in Bicuspid Valves
There are caveats to this new registry analysis, but mortality and stroke rates were in line with TAVR outcomes in tricuspid AS.
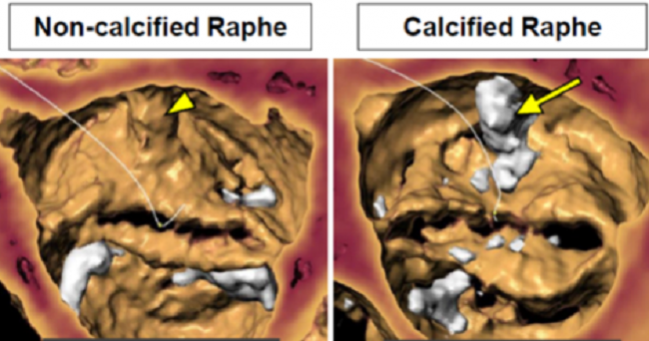
A deep registry dive provides more evidence that patients with bicuspid aortic stenosis at increased risk for surgery can safely undergo transcatheter aortic valve replacement, with results showing comparable early clinical outcomes compared to patients who have trileaflet stenosis treated with the same approach.
In an analysis of the Society of Thoracic Surgeons/American College of Cardiology TVT Registry, patients with bicuspid aortic stenosis treated with Evolut R or Evolut PRO (Medtronic) valves had equivalent risks of death and stroke at 30 days and 1 year when compared with patients with tricuspid aortic stenosis given the same the self-expanding valves.
“There’s always some selection bias in terms of who physicians are willing to put into a study or in whom physicians are even willing to do the procedure to begin with, and that’s a very difficult thing to tease out,” lead investigator John Forrest, MD (Yale University School of Medicine, New Haven, CT), told TCTMD. “But I do think we’ve developed a better understanding of aortic valve disease and anatomies that are conducive to transcatheter therapies, as well as anatomies not so conducive. What this study showed is that when you look at what’s presently being done for patients with bicuspid aortic valve disease undergoing TAVR within the TVT registry, the outcomes are fairly similar to that of people who are getting TAVR for tricuspid aortic stenosis.”
Toby Rogers, MD, PhD (MedStar Heart & Vascular Institute, Washington, DC), who along with Ron Waksman, MD (MedStar Heart & Vascular Institute), conducted a small feasibility study of TAVR in low-risk patients with bicuspid aortic stenosis, said the results from the ACC/STS TVT registry look quite promising.
“The results are overall very encouraging,” he told TCTMD. “The procedural outcomes support that TAVR is safe in bicuspid patients.”
Published online recently in JACC: Cardiovascular Interventions, the new findings line up nicely with several other reports, including the LRT Bicuspid Study, the Evolut Low-Risk Bicuspid study, and an analysis of the STS/ACC TVT registry presented last year by Raj Makkar, MD (Cedars-Sinai Medical Center, Los Angeles, CA), that looked at clinical outcomes of patients with bicuspid aortic stenosis treated with the Sapien 3 (Edwards Lifesciences) transcatheter valve.
In that latter analysis, though, investigators observed a higher risk of stroke at 30 days in patients with bicuspid valves treated with TAVR compared with transcatheter replacement in patients with a tricuspid valve. There was no difference in the risk of stroke at 1 year. Rogers noted that bicuspid aortic valves tend to more calcified compared with tricuspid valves so there is an expectation that more debris would be released during TAVR. Much of the present analysis period, Rogers noted, predated the approval of Sentinel (Boston Scientific), a cerebral embolic protection device.
Bicuspid Patients Present Earlier
The latest STS/ACC TVT registry analysis included 932 patients with bicuspid aortic stenosis treated with the self-expanding Evolut R/PRO between 2015 and 2018. The propensity-matched analysis included 26,154 patients with tricuspid valve morphology who underwent TAVR during the same period, which led to 929 matched patients. Before adjustment, patients with bicuspid aortic stenosis were nearly 8 years younger, more frequently male, and had a lower STS-PROM score.
In-hospital events, including mortality, stroke, coronary obstruction, pacemaker implantations, vascular complications, and post-procedural length of stay did not differ between the two groups. At 30 days and 1 year, clinical outcomes remained similar, with the exception of reinterventions which were higher at both timepoints for bicuspid patients.
STS/ACC TVT Registry: Clinical Outcomes With Evolut R and Evolut PRO
|
|
Bicuspid Valve |
Tricuspid Valve |
P Value |
|
All-Cause Mortality 30 Days 1 Year |
2.6% 10.4% |
1.7% 12.4% |
0.18 0.63 |
|
Stroke 30 Days 1 Year |
3.4% 3.9% |
2.7% 4.4% |
0.41 0.93 |
|
Pacemaker Implantation 30 Days 1 Year |
15.4% 16.4% |
13.7% 15.9% |
0.30 0.52 |
|
Aortic Valve Reintervention 30 Days 1 Year |
0.8% 1.7%
|
0.1% 0.3% |
0.03 0.01 |
|
Valve-Related Readmission 30 Days 1 Year |
1.1% 3.8% |
0.7% 3.1% |
0.31 0.40 |
To TCTMD, Forrest said some of the early-generation TAVR devices used in the treatment of patients with bicuspid aortic stenosis had results that were “less than optimal.” The present analysis, he said, was designed to understand the risks associated with TAVR in this patient population, and to determine if a patient with a bicuspid valve may be at higher risk of stroke when implanted with a transcatheter valve. He noted that a self-expanding prothesis tends to “take the shape” of the annulus, rather than reshaping the annulus into a circle like the balloon-expandable valve.
“At higher risk, you might say, ‘Well, if you can get a reasonable result with TAVR, then go ahead,’” said Tang. “It can lead to faster recovery and so on, but with younger patients we really need to be careful about procedures with less than surgical-like results.”
Tang also noted that the Evolut Low-Risk Bicuspid Study, which was presented in March 2020 during the American College of Cardiology’s virtual late-breaking clinical sessions, showed there was a significant number of patients who had mild or greater paravalvular leak and needed a permanent pacemaker, just as there was in this registry analysis. This is a concern given that operators already selected patients with the best possible anatomy for the transcatheter procedure. In these younger, low-risk patients with bicuspid valves, the annulus is typically large with generous root anatomy, meaning that valve-in-valve TAVR after a failed surgical valve is typically not an issue.
“Surgery may be a good first step to manage these patients with few comorbidities,” he said.
Moreover, the Ross procedure, which involves pulmonary autograft replacement, might be a suitable option in young and middle-aged adults with bicuspid aortic stenosis rather than conventional aortic valve replacement, said Tang. In the hands of an experienced, high-volume surgeon, the Ross procedure can yield excellent clinical outcomes, with patients living as long as those without cardiac disease.
What Type of Bicuspid Valve?
One of the biggest limitations of the TVT Registry analysis is the absence of information on valve phenotypes, specifically Sievers classification. Rogers said their study of TAVR in low-risk patients with bicuspid valves, as well as the Evolut Low-Risk Bicuspid Study, included only a minority of patients with “true” Sievers type 0 bicuspid valves. In the TVT Registry, the patients were older, and Rogers said it’s not unreasonable to assume that most patients had a Sievers type 1 or 2 valve given their age.
“I think we all believe that Sievers type 0, which is a true bicuspid valve with just two leaflets, behaves differently than Sievers type 1, which is three leaflets with two fused together,” said Rogers. “That, to me, is one of the strengths of the two low-risk cohorts in that all patients had a CT scan beforehand, which was adjudicated, and we know the bicuspid morphology. In those studies, the majority of patients were type 1, and only a minority had a Sievers type 0 valve.”
The bottom line, Rogers continued, is that not all bicuspid valves should be lumped together. “I don’t think we should interpret these excellent [TVT Registry] results as a greenlight for every bicuspid patient to have TAVR,” he cautioned, “because we simply don’t have enough data in those younger, Sievers type 0 patients, who probably weren’t represented in this cohort and comprised a minority in the two low-risk bicuspid studies.”
Forrest agreed that the lack of information on valve morphology is a limitation.
“Within the TVT Registry, it doesn’t ask the type of bicuspid valve, which stands a little bit in contrast to the STS surgical database, which does ask for a breakdown in terms of Sievers classifications,” he said. “We don’t get that with the TVT Registry, and I think it’s something we might consider, especially as TAVR is done for frequently for younger, lower-risk patients, some of whom are going to have bicuspid aortic valve disease.”
Forrest added that there is a wide spectrum of aortic valves, even among those with the same Sievers classification. Some type 1 bicuspid valves might be layered with extensive calcification while others may not, for example. Within his clinical practice, he has historically had reservations about implanting a transcatheter heart valve in a Sievers type 0 bicuspid valve, although his perspective is changing. He noted that the Evolut Low-Risk Bicuspid Study, which he was involved in, didn’t show a difference in clinical outcomes by Sievers classification.
Rather than focus on valve morphology, the decision to intervene with TAVR in a bicuspid aortic valve might be best judged by other factors, such as the extent of calcification. In an editorial, Didier Tchetche, MD, and Saifullah Siddiqui, MD (Clinique Pasteur, Toulouse, France), state that determining who is a good candidate for TAVR is an open question, wondering whether root anatomy should be the main factor or if age should dictate treatment in bicuspid aortic valve disease. With younger patients, durability of the transcatheter valves is a big unknown.
Is a Randomized Trial Needed?
One consistent debate surrounding the optimal treatment of bicuspid aortic stenosis is whether a randomized controlled trial is necessary to prove that TAVR is a safe and effective option. For Forrest, whether such a trial needs to be done depends on the question the trial is designed to answer.
“If the goal is not to necessarily change approval, but to change the guidelines—that is, to say this is what we should be doing—then we have pretty strong precedent that guidelines should be set by randomized studies,” he said.
For such a trial, Forrest believes the comparator arm should be surgical aortic valve replacement and follow-up should extend beyond 1 year, something Tchetche and Siddiqui agree with in their editorial. Funding for such a trial would be extremely hard to come by, though, especially since industry is likely unwilling to pay for a clinical trial designed to change guidelines.
Proof of safety and feasibility is something different. “If we’re just looking for evidence to say, ‘Yes, you can use TAVR in [bicuspid] cases,’ then I do think there is a growing evidence base, certainly in patients at increased risk for surgery, to show that it works well in these patients,” said Forrest.
Photo Credit: Guadagnoli AF. Bicuspid valve TAVI: as safe as tricuspid valve intervention? Presented at: TCT 2019. September 28, 2019. San Francisco, CA.
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Forrest JK, Kaple RK, Ramlawi B, et al. Transcatheter aortic valve replacement in bicuspid vs tricuspid aortic valves from the STS/ACC TVT Registry. J Am Coll Cardiol Intv. 2020;Epub ahead of print.
Tchetche D, Siddiqui S. Percutaneous management of bicuspid aortic valves: still remaining questions. J Am Coll Cardiol Intv. 2020;Epub ahead of print.
Disclosures
- Forrest reports grant support/research contracts and consulting, honoraria, and speaking fees from Edwards Lifesciences and Medtronic.
- Tchetche and Siddiqui report no relevant conflicts of interest.
- Rogers reports consulting/proctoring for Edwards Lifesciences and Medtronic.
- Tang reports serving as a physician proctor for Edwards Lifesciences and Medtronic as well as consulting for Medtronic.


Comments