Harmony TPV Continues to Reduce Pulmonary Valve Regurgitation at 1 Year
The platform provides an opportunity to reduce the lifetime open heart surgery burden these patients face, says Thomas K. Jones.
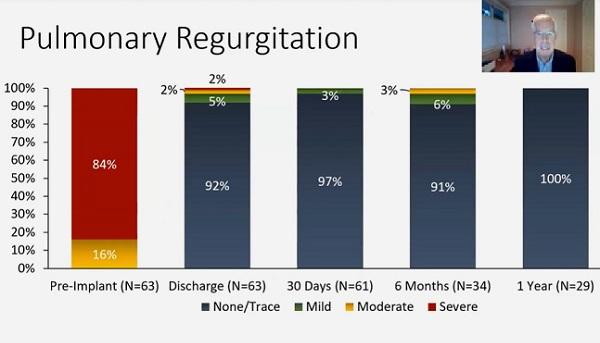
Updated data from the Harmony transcatheter pulmonary valve (TPV) pivotal and continued access studies show sustained safety and efficacy of the device through 1 year in a cohort of patients with no other transcatheter options.
The findings come on the heels of the US Food and Drug Administration approving the Harmony system (Medtronic) in March as the first nonsurgical heart valve to treat pulmonary valve regurgitation in pediatric and adult patients with a native or surgically repaired right ventricular outflow tract (RVOT). It is intended for patients with valve disease secondary to congenital heart disease (CHD). Six-month results for the Harmony TPV were released during last year’s Society for Cardiovascular Angiography and Interventions (SCAI) meeting.
“Unlike any other TPV, this novel technology is designed to expand into the enlarged RVOT in these patients while simultaneously deploying a suitable bioprosthetic pulmonary valve,” Thomas K. Jones, MD (Seattle Children’s Hospital, WA), who presented the latest findings this week at SCAI 2021, said in a press release. “The Harmony TPV system has the potential to fundamentally alter the lifetime management of CHD patients from here on out.”
Before this valve became available, patients with significant pulmonary valve regurgitation, RV dysfunction, and symptoms were referred for surgical pulmonary valve replacement or a homograft RVOT conduit implantation, Jones explained in a press briefing prior to SCAI. “This remains a viably important therapy, particularly in the younger patient population,” he told the media. “But in the adolescent and adult population, these are all patients that have undergone prior open-heart surgical care, and so having a transcatheter alternative such as the Harmony valve is very well embraced by both referring physicians and patients.”
Long Time Frame Imagined
The additional data include 67 patients who were successfully implanted with the Harmony valve and discharged—31 in the pivotal trial with at least 1 year of follow-up (mean 21.7 months) and 36 in the continued access study.
At 6 months, 91% of patients reported none or trace pulmonary valve regurgitation, while 6% reported mild and 3% reported moderate. By 1 year, however, pulmonary valve regurgitation was nonexistent or trace in all patients. Similarly, paravalvular leak was none/trace in 94% patients at 6 months and in 100% at 1 year.
Clinical outcomes have been good through 1 year with the most common events being ventricular tachycardia (19%) and ventricular premature beats (10%). No deaths or cases of endocarditis, stent fracture, embolization, thrombosis, or MI have been reported and no surgical explants or repairs of the device or RVOT have been required through 1 year. During the time of their initial procedure, one patient underwent a valve-in-valve implantation of the Melody device (Medtronic) subsequent to receiving Harmony and another underwent balloon angioplasty of the distal device; both are still doing well, according to Jones.
Our hope is that 20, 30 years, or perhaps longer might be achievable with this technology. Thomas K. Jones
The researchers plan to follow all patients through 10 years. The Harmony device, which likely will have as good durability as existing transcatheter valves, leaves open the opportunity for future valve-in-valve procedures down the line, said Jones.
“These patients have had this ongoing burden and ongoing risk of repeat surgeries through their lifetime to replace these dysfunctional valves,” Jones told TCTMD.
“This platform allows us to avoid that very first open-heart operation to put in a pulmonary valve. Instead, we deliver the Harmony valve, and then in the future this valve platform with its expandable nitinol frame can accommodate at least one and probably more transcatheter valves,” he said. “It offers the opportunity for a patient who needs a pulmonary valve to have a Harmony valve implanted and then perhaps go another 10 years or more with that valve and then undergo a transcatheter valve after that. Our hope is that 20, 30 years, or perhaps longer might be achievable with this technology.”
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Jones TK. The Harmony transcatheter pulmonary valve: 1-year outcomes from the pivotal trial and 30-day outcomes from the continued access study. Presented at: SCAI 2021. April 30, 2021.
Disclosures
- Jones reports receiving research grant support and serving as an investigator, consultant, and proctor for Medtronic and Edwards Lifesciences.



Comments