A Hole in the Heart for HFpEF? Setbacks, Progress for Interatrial Shunts
REDUCE-LAP HF II dampened some hopes for LA shunts in heart failure, but the field is learning lessons and moving forward.

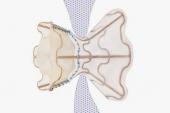
Heart failure with preserved ejection fraction (HFpEF) can arise from a range of genetic and physiological triggers, but the common denominator—elevated left atrial (LA) pressure—has spawned a host of investigational interatrial shunt devices aimed at LA decompression. After nearly a decade of research, most single-arm studies have showed improvements in exercise capacity and quality of life, along with reductions in pulmonary capillary wedge pressure (PCWP) at rest or during exercise.
The first randomized, controlled trial in this space, however, showed no benefit to shunt implantation versus a sham procedure, begging the question: is hope still afloat for the theory that making a hole in the heart can help in HFpEF?
A flurry of recent studies imply that the field is forging on. The EuroPCR, European Society of Cardiology Heart Failure (ESC-HF), Society for Cardiovascular Angiography and Interventions (SCAI), and TVT meetings this spring all featured new study results, new technologies, or updated analyses from prior presentations and publications. That’s despite the fact that REDUCE LAP-HF II, the largest trial to date, testing the Corvia interatrial shunt in HFpEF, came up short. The trial was presented at THT 2022 in February and published simultaneously in the Lancet.
“I think there's still a lot of excitement,” the lead investigator for REDUCE LAP-HF II, Sanjiv Shah, MD (Northwestern University Feinberg School of Medicine, Chicago, IL), told TCTMD, citing examples of other, earlier-stage innovations and approaches that are moving forward. “There's still a lot of interest and we're just going to have to see,” he said.
While most of the studies to date have been small, single-arm series with surrogate endpoints, results have been more or less consistent in demonstrating that the procedure is safe, reliably reduces LA pressures, and improves exercise capacity, Shah noted. There remains a lot of industry investment in the field, as well, he said.
There's still a lot of interest and we're just going to have to see. Sanjiv Shah
The idea that an iatrogenic shunt could be helpful in this setting stems from the fact that elevated LA pressure tends to cause similar symptoms in HFpEF as it does in mitral stenosis, as Lars Lund, MD, PhD (Karolinska Institutet, Stockholm, Sweden), explained to TCTMD. More than a century ago, researchers noted that mitral stenosis progression tended to be delayed or attenuated in patients who also had a congenital atrial septal defect. That led to the theory that creating a hole in the septum of heart failure patients with elevated LA pressures might also alleviate their heart failure symptoms.
Daniel Burkhoff, MD, PhD (Cardiovascular Research foundation, New York, NY), who has also been involved in the REDUCE-LAP HF trials with the Corvia device, estimates that there are at least seven companies working in this space—four developing implantable devices (Corvia, V-Wave, Edwards Lifesciences, and Occlutech) and three working on shunt-creation approaches that leave no device behind (Alleviant, NoYa, and InterShunt).
The Edwards and Occlutech devices are both in early feasibility studies, ALt FLOW US (55 patients) and PRELIEVE (106 patients), both with estimated completion dates in 2022.
“I’ve found it encouraging that most of the projects I'm aware of are continuing,” Burkhoff said. “There are none that I've heard are stopping. Enrollment continues in other studies aside from the Corvia study.” The farthest along, he noted, is RELIEVE-HF, testing V-Wave’s Ventura interatrial shunt device across the spectrum of ejection fraction. The study aims to enroll 500 patients at 120 sites internationally, with a plan to scale up to 1,000 patients based on interim analysis findings.
“It's almost as though the Corvia study had minimal impact on the momentum, at least for the companies, to continue,” Burkhoff said.
Corvia’s Setback
As reported by TCTMD, REDUCE LAP-HF II, conducted at 89 sites internationally, randomized 626 patients (out of 1,072 screened) to an atrial shunt device or a sham procedure. All patients had to have an ejection fraction of 40% or higher and exercise PCWP ≥ 25 mm Hg while exceeding right atrial pressure by at least 5 mm Hg. Patients with right ventricular enlargement or dysfunction were excluded.
At 12 months, there was no significant difference in the cumulative rate of cardiovascular death or stroke between groups, although it was numerically lower in the sham-treated patients. By 24 months, however, there was no difference in cumulative rates of heart failure events or a composite safety endpoint, but patients in the shunt group had experienced more cardiac events than those in the sham: 4% versus 1% (P = 0.025).
Delving deeper into this signal, investigators found that patients who did not have evidence of latent pulmonary vascular disease (PVD) based on invasive exercise hemodynamics appeared to benefit from shunt placement, whereas those with latent PVD fared worse. Patients with prior pacemakers also appeared to do poorly with shunt placement.
“The pacemaker is almost like a sign that the right heart is sick and even if it didn't show up on the echo, even if patients met the inclusion criteria, that right ventricle is more vulnerable and they do worse, at least with the Corvia device,” Shah explained.
As a result, he added, patients with pacemakers will be excluded in the next Corvia trial.
Gaining More Insights
Whether and how much the presence of PVD can or should be used to define the patients best suited to this procedure is unclear. An unblinded snapshot of patients participating in the nonrandomized roll-in phase of the RELIEVE-HF trial of the V-Wave device, also presented at THT 2022 back in February, suggest that shunt size—potentially in relation to PVD—may also be key. RELIEVE-HF investigators at THT were quick to point out that the shunt created with the V-Wave is smaller than that of Corvia (5 mm vs 8 mm), which might help explain their promising, albeit unblinded, early results.
There are many patients who subjectively suffer a lot from their poor quality of life and functional capacity. Lars Lund
That theory was further explored in a presentation by Julio Nuñez Villota, MD, PhD (University of Valencia, Spain), at the ESC-HF meeting in May. In an analysis derived from echocardiographic data, investigators estimated that the 5-mm shunt produced by the V-Wave creates “just the right” amount of shunt flow, said Nuñez Villota in Madrid, decompressing the left atrium without adverse effects on the right heart or pulmonary circulation. By contrast, he explained, an 8-mm shunt leads to double the amount of shunt flow at any gradient, “with deleterious effects on right ventricle function.”
Patients treated with the V-Wave shunt, he noted, also showed significant gains in left ventricular ejection fraction at 1 year, as well as improvements in RV systolic function and left and right ventricle dimensions.
“To our delight, we found multiple indicators that the right ventricle was actually getting better,” Nuñez Villota is quoted in a V-Wave press release summarizing the ESC-HF presentation. “This may be clinically important considering the recent negative REDUCE-LAP-HF II trial that used a different and larger 8-mm interatrial shunt that has about twice the flow as the 5-mm V-Wave Ventura Shunt.”
RELIEVE-HF, said Nuñez Villota, is expected to finish recruitment this fall.
The Ideal Patient
What’s clear is that the accumulating data are carving away at the patient population best suited to this type of device therapy.
“You really cannot have right-sided heart failure at all, if you're going to benefit from this intervention, because the increased flow of blood to the right is going to compromise your right-sided function further,” Lund told TCTMD. Add to this the emergence of latent pulmonary vascular disease and pulmonary resistance as a contraindication, not to mention the dearth of centers capable of performing the invasive tests required to definitively diagnose these conditions, and the pool of patients shrinks further.
“That’s a further subgroup of a subgroup of a subgroup, so to speak,” Lund said. “Going forward, companies need to pick patients who have a normal or good right-sided function at rest. That was already known, and that still applies. Also, patients who do not have resistance in the lungs at rest: that was known, and that still applies. But thirdly, they need to pick patients who also do not get increased resistance during exercise and that was previously not known, but that is known now as a result of [REDUCE-LAP HF II].”
Investigators moving into next stage testing will likely consider all of these factors in refining their upcoming or ongoing clinical trials, Lund added.
Worth noting, said Burkhoff, is that V-Wave is not only focused exclusively on HFpEF patients, but “has not embraced exercise testing” as part of its clinical trial/patient selection process.
“I suspect if they have a negative trial like Corvia’s, they will be able to find another subgroup that doesn't rely on that, that's just the way subgroup analyses work,” he said. “They'll find some resting or other noninvasive thing that also correlates with outcome.”
And whether that, in turn, proves to be a good or comparable way to hone patient selection will again need confirmation in further studies.
Shah pointed out that even if the patient population shrinks, it is by no means small. In REDUCE-LAP HF II, even after excluding patients with a pulmonary vascular resistance greater than 1.74 Wood units at peak exercise and/or a pacemaker, that still leaves more than 50% of patients eligible.
“People might say, well that’s just a sliver of the population, but it turns out it's about 2 million people in the US that fit the inclusion criteria for the next trial. So this still could have a major benefit for a lot of patients,” Shah argued. “And who's to say, it could be that in patients with an exercise pulmonary vascular resistance above 1.74, we might be able to treat them with pulmonary vasodilators like sildenafil to get that number down and then they'll be candidates for the device, potentially.”
Whether that population is sizeable enough to be attractive to the companies trying to bring these shunts to market is another thing entirely, Lund noted.
“If indeed a future trial showed that a subgroup of a subgroup of a subgroup benefits from this treatment, the implementation is going is still going to be limited,” Lund said. “But it's such a large population and there are many patients who subjectively suffer a lot from their poor quality of life and functional capacity. . . . I don't think any investigator or sponsor or company anticipates that this is going to be a large [market], but I think many do consider the possibility that it'll be a small and specific item that helps a not insignificant proportion of patients.”
The question of ideal shunt size, device, or technique also awaits more data. At ESC-HF in May, Lund presented early results with Alleviant Medical’s no-implant interatrial shunt technology in the ALLEVIATE-HF 1 and 2 first-in-human, nonrandomized trials. The same data were presented by Peter Fail, MD (Cardiovascular Institute of the South, Houma, LA), at TVT 2022 in June.
As both Lund and Fail showed in their presentations, patients treated with the Alleviant no-shunt technique had statistically significant improvement in peak exercise PCWP and NT-proBNP and sustained improvement in symptoms and NYHA functional class. The system excises tissue via mechanical compression and a short pulse of radiofrequency energy to create a roughly 7-mm shunt with smooth, rounded edges, stable patency, and uniform endothelialization.
Investigators are moving forward with a pivotal study, Lund said.
Next Horizons
For now, all eyes will be watching for the randomized V-Wave results, anticipated for late 2022 or early 2023. If positive, the narrow inclusion criteria implied by the REDUCE-LAP HF II findings will be revisited, particularly given the inclusion of HFrEF patients in the study.
According to Shah, the “truly HFpEF” patients in REDUCE-LAP HF II—those with ejection fractions greater than 50%—appear to have gleaned the most benefit from a shunt.
“I think it will be really interesting to see, throughout the full spectrum of EF, does it work better in HFpEF or HFrEF or somewhere in the middle? That still needs to be determined,” Shah said, “but I do think that it's going to be tough to do studies in HFrEF and even moderately reduced EF, because there are so many medical options already.”
It’s also not clear when in the disease process an interatrial shunt might work best.
“Normally later-stage patients, once they start progressing, the right heart gets sicker, the pulmonary vascular resistance goes up, so you can't wait too long. In that sense, earlier may be better,” Shah speculated. “On the other hand, typically we reserve devices for later on, when somebody has failed medical therapy. So I think there's going to be a sweet spot for devices like this, where patients are not too sick in right heart failure, but you've tried SGLT2 inhibitors, you’ve tried mineral corticoid receptor antagonists, and they’re still struggling.”
It's almost as though the Corvia study had minimal impact on the momentum, at least for the companies, to continue. Daniel Burkhoff
At that point, he continued, “if you do a right heart catheterization and their pulmonary capillary wedge pressure at rest is normal, but it goes way up with exercise, medically there's nothing we have for treatment. . . . That’s the kind of patient that I think we really struggle with, that could potentially benefit from a shunt device.”
For the large majority of heart failure specialists more comfortable with drug therapies than with interventional devices, interatrial shunts have a long road to travel. Lund’s prediction is that with the right, highly selected trial population, interatrial shunts will likely prove to enhance quality of life and functional capacity but not have an impact on heart failure hospitalizations or death. Regulators, he noted, are increasingly open to accepting patient-centered endpoints in trials, so this may not be a barrier.
Reimbursement may be a separate hurdle, particularly in places like the United States, he predicted. Yet this may ultimately help ensure that only patients who meet the strict conditions established by trials end up being the ones who get the devices.
The biggest stumbling block, he said, may be among the heart failure doctors themselves.
“There needs to be awareness and willingness and capabilities, broadly in the community, and I think that that will also exist but be very limited,” said Lund. “My prediction is that this approach will become clinically available, approved, and reimbursed, but will be utilized poorly or little and that will be a concern for the companies.”
Burkhoff said REDUCE-LAP II was undoubtedly “a setback, and there were certainly some in the heart failure community at large that did not think this would work, from the beginning, and obviously this reinforces their thinking.”
But for now, physicians eager for new therapies, particularly for HFpEF patients are, appropriately, awaiting more data. “The heart failure community in general is very conservative,” Burkhoff said. “They are willing to participate in clinical trials, [but] they are reticent to endorse or express premature enthusiasm, for any therapy.”
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioDisclosures
- Shah reports receiving research funding from Actelion, AstraZeneca, Corvia, Novartis, and Pfizer and serving on consulting/advisory board/steering committees for Abbott, Actelion, AstraZeneca, Amgen, Aria CV, Axon, Bayer, Bristol Myers Squibb, Boehringer-Ingelheim, Cardiora, Coridea, CVRx, Cyclerion, Cytokinetics, Edwards, Eisai, Imara, Intellia, Ionis, Keyto, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sanofi, Shifamed, Tenax, Tenaya, and United Therapeutics.
- Burkhoff reports grant/research support from Abiomed, Ancora, and Edwards Life Science, and Axon Therapies and consulting fees/honoraria from BackBeat/Orchestra, BioMind, CardioDyme, Corvia, HELP Therapeutics, IMPULSE Dynamics, and PVLoops LLC.
- Lund reports serving on the steering committee and receiving consulting fees from Alleviant.


Comments