Low-Attenuation Plaque Volume Predicts MI in SCOT-HEART Analysis
Burden of this high-risk plaque performed better than risk scores, coronary calcium, and obstructive CAD at predicting risk.
Using coronary CT angiography to assess plaque burden—and specifically the burden of “low-attenuation” plaque—in patients presenting with chest pain can help predict the risk of future myocardial infarction, according to a post hoc analysis of the SCOT-HEART trial.
Compared with other more commonly used predictors including a CV risk score, coronary artery calcium (CAC), Agatston units, and presence of obstructive coronary artery disease, the burden of low-attenuation plaque proved to be the best predictor of later events, senior author Marc Dweck, MBChB, PhD (University of Edinburgh, Scotland), told TCTMD.
“But probably all of the previous tests have kind of measured the wrong type of plaque,” he pointed out. “CT calcium scoring, for example, measures calcified plaques, which are the ones unlikely to rupture, so plaque type matters as well. What this new analysis is doing is measuring, directly, the burden of the plaque that causes heart attacks: the low-attenuation plaque that is associated with a large, necrotic lipid core.”
The paper by Dweck and colleagues, with first author Michelle C. Williams, MBChB, PhD (University of Edinburgh), was originally accepted for presentation at the American College of Cardiology 2020 Scientific Session and was published March 16, 2020, in Circulation.
Predictive Plaques
As previously reported by TCTMD, the randomized SCOT-HEART trial showed a 41% reduction in the endpoint of coronary heart disease death or nonfatal MI at 5 years when coronary CT angiography was used instead of standard care, predominantly stress testing, to guide the management of patients presenting with chest pain.
Low-attenuation noncalcified plaque quantified from a standard test (CTA) predicts future MI better than traditional risk scoring, in prospective multicenter SCOT-HEART trial, in Circulation today @imagingmedsci @MarcDweck @Heart_SCCT https://t.co/Rx5zhhyWtC pic.twitter.com/4LRHoGLoxc
— Damini Dey (@damini_dey) March 16, 2020
For the current analysis, investigators performed noncontrast, electrocardiogram-gated CT for calcium scoring and contrast-enhanced electrocardiogram-gated coronary CT angiography in all SCOT-HEART patients at baseline. Calcium scores were calculated using established methods and stenoses were assessed by trained observers, with obstructive coronary artery disease defined as the presence of one or more epicardial vessels with a stenosis of 50% or greater in the left main stem or 70% or greater in other segments. The ASSIGN score, derived from the Framingham score to apply to the Scottish population, was used for cardiovascular risk prediction.
Plaque volume was assessed using semiautomatic software in any arterial segments identified as having nonobstructive or obstructive disease and graded as total plaque, calcified plaque, noncalcified plaque, and low-attenuation plaque, with each expressed as a percentage of the relevant plaque volume relative to the total assessed volume.
In all, 1,769 patients were followed for a median of 4.7 years; 41 had a fatal or nonfatal MI over follow-up. In the cohort as a whole, low-attenuation plaque burden correlated highly with coronary artery calcium score, weakly with cardiovascular risk scores, and very strongly with severity of luminal coronary stenosis. When it came to predicting risk of subsequent MI, low-attenuation plaque burden emerged as the best (HR 1.60 per doubling; 95% CI 1.10-2.34) and added incrementally to the predictive power of CAC score, CV risk score, and presence of obstructive coronary disease. Patients with a low-attenuation plaque burden of more than 4% were five times more likely to suffer a fatal or nonfatal MI.
The aftermarket software used to calculate volume of low-attenuation plaques in this analysis was a program called Autoplaque, developed by study co-author Damini Dey, PhD (Cedars-Sinai Medical Center, Los Angeles, CA), but other add-on software options are available, Dweck noted.
“Now that we have software that does this reliably, we can measure the burden of different types of plaque,” he said. And while the SCOT-HEART analysis findings now need to be validated, “the good news is we have all these data sets from different studies where we can go back and look at this.”
Commenting on the study for TCTMD, Jagat Narula, MD, PhD (Mount Sinai Hospital, New York, NY), noted that there are a range of software options for plaque characterization “in the commercial and investigational space,” but said these have produced “widely variable results” so far.
“The directionality of these programs, even though the same, [have accrued] a range of numbers [that] are not mostly in agreement,” he said in an email. “We need to resolve the differences by multipronged efforts. Although plaque analysis algorithms could eventually emerge as a promising tool, it would need effective collaboration amongst numerous vendors to come to a consensus. The software is not ready to be used on day-to-day basis and should only be considered a research tool. We would also need a similar effort for various software programs, and would need them to agree (not only correlate) with the pathological gold standard.”
For now, Dweck suggested the PROMISE trial and the CT CONFIRM registry as ideal places to start in terms of validating the accuracy of the algorithm used in the current analysis, as well as the recent ISCHEMIA trial, in which all patients had a baseline CT.
Indeed, the ISCHEMIA trial was designed, in part, to understand whether presence of ischemia was predictive of later events and the ability of different interventions to reduce that risk.
“We have these excellent interventions to reduce ischemia, and they don’t seem to reduce your risk of an MI,” Dweck said. “That suggests that there is not a causal relationship between ischemia and heart attack. My personal interpretation is that ischemia is a surrogate for plaque burden. Therefore, if you are a patient with ischemia, you'll have a lot of plaque and a lot of low-attenuation plaque, thereby explaining your increased MI risk. In this preliminary study, that’s kind of borne out. If you have obstructive CAD, then you’re at higher risk of a heart attack, but in the multivariate model, that effect is lost when low-attenuation plaque is added into the model.”
My personal interpretation is that ischemia is a surrogate for plaque burden. Marc Dweck
The focus on adverse plaque burden, rather than the identification of a specific “vulnerable” plaque, also shifts the emphasis of interventions away from an approach like prophylactic stenting and towards systemic therapies—both medical and lifestyle, Dweck said. “Having an assessment of how much adverse plaque you have would allow [physicians] to uptitrate those individualized interventions to the individual patient.”
Narula pointed out that the published ISCHEMIA results are due out any day and should “provide some missing pieces.”
Currently, he said, “SCOT-HEART is one of the most important investigations that we have witnessed in the recent history of clinical studies and has contributed to amendments in guidelines for the management of stable coronary disease.” Unfortunately SCOT-HEART, which was designed as a pragmatic study, did not try “to clarify the individual role of the extent of luminal stenosis, its functional significance (ie, FFR or myocardial ischemia) and the pathological characterization of the vessel wall, and whether one or more of the anatomical, physiological, or pathological traits would allow superior decision-making for revascularization in stable coronary artery disease,” Narula noted.
“Furthermore, it is not quite possible to identify plaque behavior by just one cross-sectional CT angiographic study,” he said. Work by Narula and colleagues has demonstrated that plaque progression is the essential but modifiable prerequisite for plaque rupture and an acute event. “It is easily possible to repeat CT imaging at a defined interval to obtain the natural history” or, ideally, use machine learning to identify “progressive plaque signatures” that could help refine the prediction of patients at risk, Narula predicted.
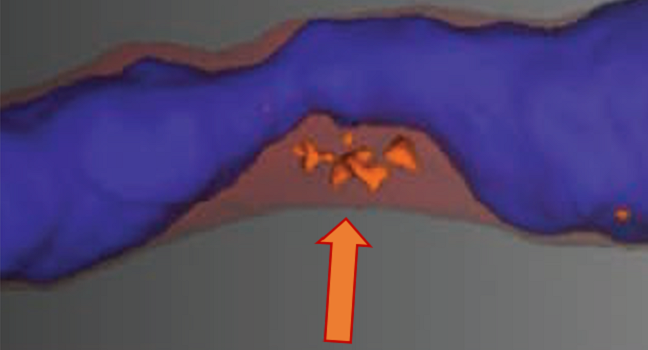
Photo Credit: Damini Dey. Image depicts a 3D atherosclerotic plaque in the mid-LAD with blue lumen, red mesh noncalcified plaque, and orange low-attenuation plaque in a 67-year-old female with atypical chest pain, who subsequently presented with acute MI.
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Williams MC, Kwiecinski J, Doris M, et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART trial. Circulation. 2020;Epub ahead of print.
Disclosures
- SCOT-HEART and several study authors were supported by the Chief Scientist Office of the Scottish Government Health and Social Care Directorates with supplementary awards from Edinburgh and Lothian’s Health Foundation Trust and the Heart Diseases Research Fund. Williams and Dweck also report support from the British Heart Foundation.
- Narula reports no relevant conflicts of interest.


Comments