Omecamtiv Mecarbil Has Biggest Benefit at Lowest EFs
That’s not surprising, Robert Harrington says, but this “helps potentially put into context how one might think about this new class of drugs.”
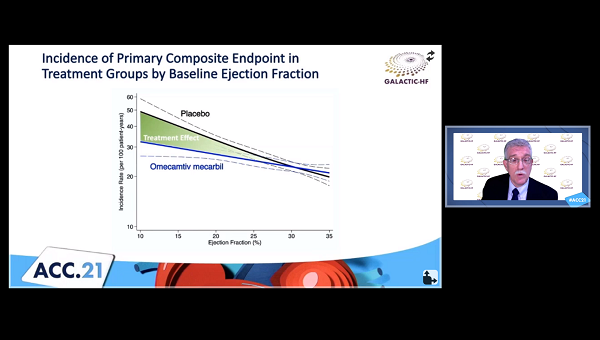
When omecamtiv mecarbil is used in patients who have chronic heart failure with reduced ejection fraction (HFrEF), the greater beneficial impact on clinical outcomes is seen in those with lower ejection fractions, a secondary analysis of the placebo-controlled GALACTIC-HF trial shows.
Among the prespecified subgroups in the trial, LVEF was the strongest modifier of the treatment effect, with larger reductions in the risk of HF events or CV death observed as values fell (P = 0.004 for interaction). Reductions in NT-proBNP were greater at the lower end of the range, as well, John Teerlink, MD (San Francisco VA Medical Center and the University of California, San Francisco), reported during the recent virtual American College of Cardiology (ACC) 2021 Scientific Session.
Omecamtiv mecarbil (Cytokinetics), a novel selective cardiac myosin activator, appeared to be safe even in the sickest patients, with no differences in serious adverse, ischemic, or arrhythmic events between the trial arms across the range of LVEF, Teerlink said. The myotrope also had no adverse effects on blood pressure, heart rate, potassium homeostasis, or renal function.
That suggests that “omecamtiv mecarbil could be readily initiated in the context of contemporary, guideline-directed medical therapies,” he told the media during a press briefing. “We believe that omecamtiv mecarbil represents a novel therapy that holds the promise of improving outcomes in patients with severely reduced ejection fraction, who are the very patients that are often the most challenging for us to treat.”
The findings were published simultaneously online in the Journal of the American College of Cardiology.
Still No Impact on Mortality
The main GALACTIC-HF results, reported in November 2020, showed that omecamtiv mecarbil reduced the composite of HF events or CV death, but the benefit was seen as modest—an absolute reduction of 2.1% and a relative reduction of 8% over a median follow-up of about 22 months—and there were no significant reductions in either individual component of that endpoint.
A prespecified subgroup analysis suggested that the benefits would be greater in patients with an LVEF of 28% or less (P = 0.003), and this new analysis presented at ACC delves further into the impact of EF. Among the 8,256 patients (mean age 66 years; 21% women) who were enrolled in the trial, mean EF at baseline was 27%. The investigators divided the participants into quartiles according to EF: ≤ 22%, 23% to 28%, 29% to 32%, and ≥ 33%.
Patients in the lowest quartile were younger on average, were less likely to be women and to be enrolled at centers in Eastern Europe/Russia, and more likely to be of nonwhite race/ethnicity. They were also less likely to have various CV comorbidities and ischemic HF etiology, with lower body mass index and systolic BP and higher heart rate, NT-proBNP, and troponin I. In terms of treatment, patients with the worst EFs were more likely to receive digoxin and ivabradine and to have implanted devices.
The treatment effect of omecamtiv mecarbil increased in a continuous fashion along with declining EF. In an analysis of LVEF quartiles, the drug reduced the risk of HF events/CV death only in the bottom two:
- ≤ 22% (HR 0.83; 95% CI 0.73-0.95)
- 23% to 28% (HR 0.85; 95% CI 0.74-0.97)
- 29% to 32% (HR 1.11; 95% CI 0.96-1.28)
- ≥ 33% (HR 0.99; 95% CI 0.84-1.16)
In the lowest quartile, the researchers calculated that 11.8 patients would need to be treated with omecamtiv mecarbil to prevent one HF event/CV death over 3 years. The drug did not reduce either CV or all-cause death, regardless of EF.
‘Highly Encouraging’ Findings
Discussing the results at the press briefing, Ileana Piña, MD (Central Michigan University, Mount Pleasant), said, “I’m so happy that you did this additional study, because how long has it been that we’ve been waiting for a drug that we can really use in this really sick population where that ejection fraction is getting lower and lower.”
She noted that these “are the people that we recommend for transplant and for mechanical assistance” in whom “we are very loathe to use inotropes, because we know that ultimately the inotrope is not going to do well.” Omecamtiv mecarbil represents an advancement because it does not increase myocardial oxygen demand and can be given in the outpatient setting, Piña said.
She indicated that she’d be interested in seeing an analyses of sex differences and potential regional differences, but said “this is just highly encouraging for that very, very sick group that we just scratch our heads and don’t know what to do with.”
Robert Harrington, MD (Stanford University, CA), a past president of the American Heart Association, said he wasn’t surprised to see that the highest-risk patients derived the greatest benefit from treatment, as that’s been seen with most other therapies. But the analysis “potentially put into context how one might think about this new class of drugs,” he told TCTMD. Subgroup analyses should be interpreted with caution, he said, but added that the careful methodology of this particular one strengthens the findings.
Cytokinetics, the drug’s developer, has said it is moving toward submission of a new drug application to the US Food and Drug Administration in the second half of 2021. When it comes to where omecamtiv mecarbil might fit in to the crowded HF drug space if it is approved, Harrington stressed that first physicians need to ensure patients are on the “foundational medicines” that have been shown to have more than a modest benefit in heart failure. The benefits of omecamtiv mecarbil were seen in patients already well treated with these agents.
“The modest effect plus the substudy effect would tell me that I would not consider this routinely for everyone with heart failure, but I would try to think about the highest-risk group of patients,” Harrington said.
Harrington said that because of how GALACTIC-HF was conducted he doesn’t have any concerns about using omecamtiv mecarbil with any of the other established heart failure therapies. “I think there’s no question that you want to be careful. It’s a whole new class of drugs, a whole new area of biology. But I think with a large trial where they’ve really carefully parsed out the safety and shown the efficacy, particularly the efficacy in the highest-risk group of patients, I’m okay with that,” he commented.
Teerlink said that there is no real suggestion that the treatment effect of omecamtiv mecarbil would be markedly different when used with newer HF agents, such as angiotensin receptor-neprilysin inhibitors (ARNIs) or sodium-glucose cotransporter 2 (SGLT2) inhibitors. In fact, he said, “there’s really every reason to believe that omecamtiv mecarbil could be complementary to those therapies. It is a very specific activator of cardiac myosin, which is in the atria, the right ventricle, and the left ventricle, and there’s no evidence of direct effects on any of the other pathways.”
Overall, Teerlink said, “I’m confident that we will be able to bring this into clinic and provide real benefit for our patients with HFrEF.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Teerlink JR, Diaz R, Felker GM, et al. Effect of ejection fraction on clinical outcomes in patients treated with omecamtiv mecarbil in GALACTIC-HF. J Am Coll Cardiol. 2021;Epub ahead of print.
Disclosures
- GALACTIC-HF was funded by Amgen, Cytokinetics, and Servier.
- Teerlink reports receiving research grants and/or consulting fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cytokinetics, Medtronic, Merck, Novartis, Servier, and Windtree Therapeutics; receiving support for attending meetings and/or travel from Abbott, Amgen, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cytokinetics, EBR Systems, LivaNova, Medtronic, Servier, and Windtree Therapeutics; and serving as treasurer for the Heart Failure Society of America.




Comments