Optimal Implantation Depth With Self-Expanding TAVR? Success May Prove Elusive
The study’s researchers call for a uniform method of assessing this parameter.
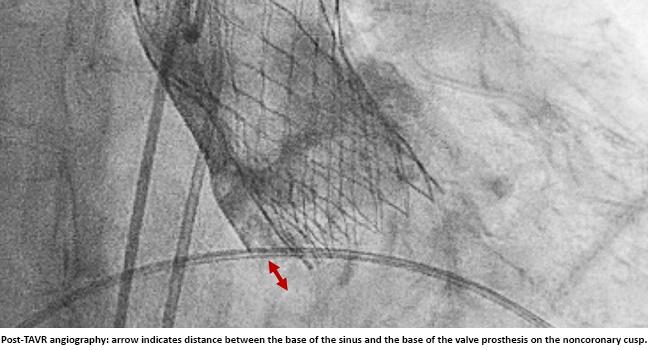
Among patients receiving self-expanding TAVR devices at a single high-volume center, the optimal implantation depth (ID) was achieved in fewer than 30%. Moreover, the lack of a standardized method for calculating ID, which can substantially influence outcomes reporting and lead to inconsistencies in perception of where the device is ultimately placed, needs to be addressed, according to researchers.
“A uniform perception of the optimal implantation depth is currently not available,” lead author Kerstin Piayda, MD (Heinrich Heine University Düsseldorf, Germany), told TCTMD in an email. “The implantation depth is often given as an average value and no statement is made in how many cases the optimal implantation depth is met. The TAVR community should find a way to translate the manufacturer’s recommendations into daily clinical practice and report the optimal implantation depth uniformly.”
James McCabe, MD (University of Washington, Seattle), who was not involved in the study, agreed that not much is known about this parameter.
“As commercialization and roll out of these technologies has evolved, I'm not sure that you're even seeing programs routinely measure pre- or post-[TAVR] hemodynamics, let alone calculate implant depths,” McCabe told TCTMD. He added that much like how the VARC definitions enabled “everyone to speak the same language,” having a universal strategy for calculating ID “could be extremely useful for clinical trials and analyses of outcomes and as an adjustment tool for pacemaker rates and things along those lines. But I'm not sure it will translate into routine use in the commercial setting.”
An ‘Omitted Parameter’
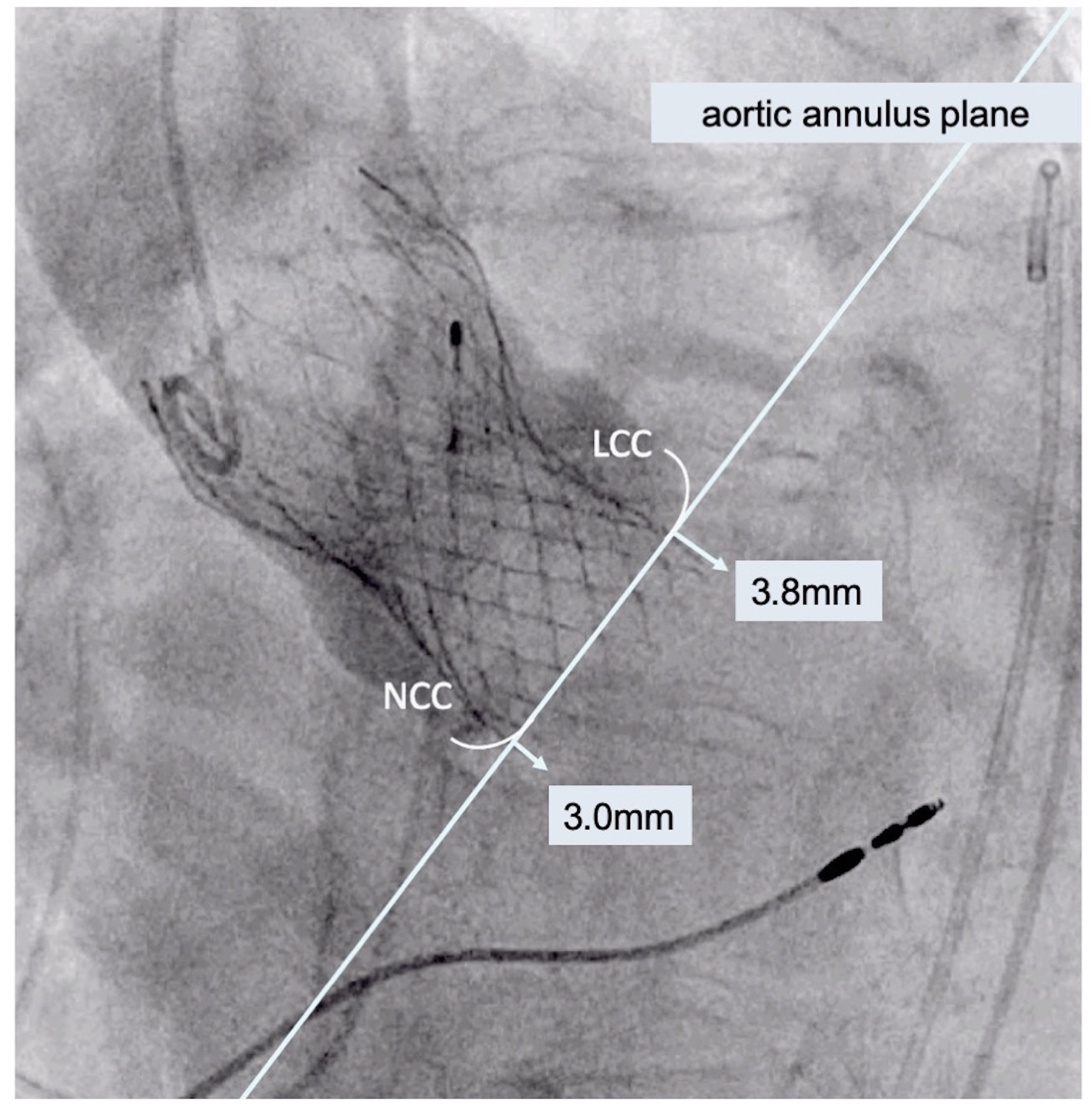
For the study, published online last week in JACC: Cardiovascular Interventions, Piayda and colleagues looked at the clinical and hemodynamic outcomes for 258 patients who received transfemoral TAVR with CoreValve Evolut R (Medtronic) at their institution between September 2015 and January 2018. The following three measurements were used to calculate ID from angiographic imaging:
- Arithmetic mean: the arithmetic mean of the measured distances from the noncoronary cusp and the left coronary cusp to the distal prosthesis end
- Noncoronary cusp distance: the distance from the noncoronary cusp to the distal prosthesis end
- Deepest edge: the deepest edge of the distal prosthesis end regardless of anatomical orientation
Resheathing or recapture of the device was necessary in 7.3% of cases, and stroke was reported in 3.9% of patients after TAVR. Additionally, new higher-grade conduction disturbances were observed in 21.3% of patients, with 12.4% receiving permanent pacemaker implantations.
The optimal ID was achieved in 25.4%, 28.4%, and 20.5% of patients using the measurements for arithmetic mean, noncoronary cusp distance, and deepest edge, respectively, and the rate of achieving the optimal ID differed depending on the method used (P = 0.008). Notably, larger prostheses were implanted deeper in the left ventricular outflow tract.
The deepest-edge method was most strongly able to differentiate the need for permanent pacemaker implantation regardless whether optimal ID was achieved or not (3.7% vs 14.6%; P = 0.033). However, the mean pressure gradient reduction after TAVR was not affected by achievement of optimal ID using this assessment (7.4 vs 8.3 mm Hg; P = 0.093).
“In the future, a uniform assessment method for the ID should be established, allowing a transparent and consistent reporting of this omitted parameter in clinical trials,” the authors write.
Universally Applicable?
“Our study is of explorative nature and is meant to direct attention to this omitted parameter, which might have significant impact on the outcome of TAVR patients,” Piayda said. “It should be seen as a starting point to find a clinical meaningful definition of the implantation depth, it’s best assessment method, and allocated endpoints. We should be open to patient tailored concepts and new ideas which should be feasible for every TAVR center in daily clinical practice.”
Physicians need to be focused on the “lifetime management” of TAVR patients, she continued. “Maybe, right at the beginning we can add a little piece to the puzzle with optimizing the concept of the ‘optimal implantation depth’ for each device in each individual patient in daily clinical practice.”
McCabe said he was surprised to see such low percentages in achieving optimal ID in this study. “Insofar as you do have the opportunities to reposition the prosthesis up to a point, you would expect that the operators would cross 50% of optimal implant depths, and so a 30% number struck me,” he said. “It's fair to point out that the implant depth is often different depending on which side of the prosthesis or which cusp you're measuring against, and we've all seen that plenty where you have a perfect implant depth on one side and simply it's never going to be perfect on the other side because there's some canting of the prosthesis relative to the annulus. . . . Nevertheless, I just somehow expected it to be a bit higher. Particularly as time has gone along, there's been plenty of learning curve time for this prosthesis.”
As for the authors’ call for a uniform method of assessing ID, McCabe said: “If the question is, ‘Is it vital to continue to evolve TAVR best practices toward optimizing outcomes?’ then I would say absolutely that is correct. However, whether or not measuring the depths is part of optimizing practice, I guess I'm not perfectly sure.”
Ultimately, he argued that “this is an important exploration of how to deploy the self-expanding prostheses with the fewest amount of recaptures and the most efficient way, but I think we'll have to see whether or not it is universally applicable to all the other self-expanders, which are either in clinical trial or are coming to bear outside the US currently and inside the US in the future.”
For example, McCabe noted that the Acurate Neo system (Boston Scientific) deploys from the top down. “Will it require some of the same strategies or different strategies? That's a little unclear,” he said. “And frankly, do you take it a step further and apply it to all TAVR prostheses in general? That I'm not sure about.”
Larger, prospective clinical trials in various devices that address the relationship between ID assessment and clinical outcome would be useful, Piayda agreed. “Moreover, implantation techniques have to be refined with the aim of reaching higher ratios of optimal implantation depth results for various devices.”
Photo Credits : Header image, James McCabe. Story image, Kerstin Piayda
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Piayda K, Hellhammer K, Veulemans V, et al. Navigating the “optimal implantation depth” with a self-expandable TAVR device in daily clinical practice. J Am Coll Cardiol Interv. 2019;Epub ahead of print.
Disclosures
- Piayda reports no relevant conflicts of interest.
- McCabe reports receiving honoraria from and consulting for Boston Scientific and Edwards Lifesciences.


Comments